
Research Article
Phys Med Rehabil Int. 2022; 9(2): 1202.
Neurophysiological Examination of the Effects of Behavioral Antecedents on Physical Balance in Older Individuals
Kodama T¹*, Abiko T¹, Matsuo N¹ and Yamaguchi H²
1Department of Physical Therapy, Kyoto Tachibana University, Japan
2Department of Advanced Development, AISIN Co., Ltd., Japan
*Corresponding author: Takayuki Kodama, Department of Physical Therapy, Faculty of Health Sciences, Kyoto Tachibana University, 34 Yamada-cho, Oyake, Yamashina-ku, Kyoto 607-8175, Japan
Received: June 30, 2022; Accepted: July 22, 2022; Published: July 29, 2022
Abstract
Falls in older individuals can be caused by balance disorders, influenced by predictive factors based on self-efficacy and outcome expectation. This study investigated the relationship between predictive factors related to regional neural functional activity and postural control. We included 16 older men (average age, 76.4±5.8 years) and evaluated their balancing ability and fall-related selfefficacy using the Japanese version of Mini-Balance Evaluation Systems Test (J-Mini-BESTest) and the Japanese version of the Falls Efficacy Scale (JFES), respectively. We performed an electroencephalogram before, during, and after postural perturbations. The cortical activity in the right Inferior Parietal Lobe (IPL) and Supplementary Motor Area (SMA) was analyzed using current density in the specific regions of interest. Foot Response Values (FRV) were used to evaluate physical responses during postural perturbations. The neural activity values in the IPL after postural perturbations indicated a significant positive correlation with JFES and J-Mini-BESTest scores when prior information was provided to participants. The neural activity values in the SMA before postural perturbations showed a significant positive correlation with J-Mini-BESTest score and a significant negative correlation with FRV. Furthermore, during postural perturbations, subjects with prior information exhibited significant positive neural correlations with neural activity between the SMA and IPL. These results suggest that neural activity in these brain regions influence balancing ability and predictive factors. Prior knowledge of a postural perturbation’s timing could be a compensatory factor promoting the activation of predictive factors.
Keywords: Postural balance; Fall-related self-efficacy; Mini-BESTest; Electroencephalography; Sense of agency; Supplementary motor area
Introduction
Quality of life in older individuals is affected by falls that occur during daily activities [1], which are more likely to happen than in younger people due to functional decline [2]. Moreover, these falls cause fractures that are significantly associated with increased mortality in this population [3]. In particular, older individuals have a high incidence of hip fractures [4], which may result in impaired activities of daily living due to limited movements like standing and walking, and a decline in functions necessary for independent living [5]. In addition, approximately half the individuals who have fallen once experience repeated falls, and 10–20% of these individuals experience a second fall in the same year [6]. Accordingly, falls in this population are considered to be a public health and social problem that may cause increased disability, morbidity, and mortality.
Balance disorders have been reported as one of the primary causes of falls in older individuals [7,8]. Indicated that self-efficacy is one of the factors that influence balance disorders caused by aging. Self-efficacy, a cognitive control system leading to self-confidence in performing a specific task [9], can be regarded as the amount of confidence an individual has in their ability to perform an action [8]. In addition, the degree of fall-related self-efficacy, which is “the degree of daily life that can be achieved without falling,” is a predictor of the fear of falling that affects posture balance [11]; however, no study has reported an association between these two factors [12]. This demonstrates that there is no unified view on the fact that fallrelated self-efficacy is a predictor of the fear of falling. Nevertheless, predictive factors that determine movements in an individual are important clues in understanding the relationship between selfefficacy and balancing ability [10].
Predictive factors consist of self-efficacy and outcome expectation, which combined, predict one’s ability to perform actions. Outcome expectations predict the outcomes of one’s own actions; [13] showed that this behavioral prediction is an important factor in balancing ability, arguing that such anticipatory mechanism helps in postural adjustment. Furthermore, the authors indicated that when a postural perturbation is experienced by the body, an anticipatory mechanism recognizes the environment and recalls a strategy to avoid falling, followed by the predictive adjustment of posture based on the recalled strategy and the actual movement. This anticipatory mechanism collects information on the environment and changes in advance, interprets how these changes affect stability based on experience, and determines an avoidance strategy. Thus, this mechanism can be viewed as “brain simulation of possible movements prior to actual movements” and has been termed prediction of behavior—the ability to collect information about the environment and to imagine possible future movements. Imagining movements does not lead to the generation of motion parameters necessary for execution (e.g., muscle recruiting and direction of movement). Rather, it refers to the ability to simulate kinesthetic sensations needed and expected for a concrete image of a movement program that can be controlled in response to a perturbation [14]. Moreover, the ability to mentally represent motor action declines with age [15-17] stated that the ability to mentally represent actions gradually decreases with age due to deterioration of motor imagery quality (i.e., isochrony between executed and imagined movements). In contrast, as a predictive factor, self-efficacy is necessary for the development of a sense of agency [18]. This development requires an active sensation (e.g., self-efficacy) of being able to act on the environment and to take a purposeful action independently to achieve an ideal or desired state [19]; a sense of agency develops when these are matched [20]. Moreover, changes in the sense of agency occur with age and are associated with changes in physical functions [21]. These findings suggest that self-efficacy may affect the physical and mental functioning in older people, and predictive factors may be closely associated with balance. If the expected outcome and self-efficacy are regarded as an image of the movement [22] and the perception of a sense of agency [23,24], respectively, the basis of brain function in creating a predictive factor of behavior that may affect balancing ability includes the neural activity of the right Inferior Parietal Lobe (IPL) and the Supplementary Motor Area (SMA). It is possible that predictive factors are established by cooperative activity between these brain regions via a neural connection.
Older individuals often experience near-falls, even if they have no experience of actual falls, due to age-related decline in sensory functioning, such as sight and hearing [25]. A decrease in attention also affects their ability to respond appropriately to changes in the environment, therefore, it is difficult for older individuals to appropriately integrate internal information such as behavioral predictive factors, and external information from the environment. To prevent falls, older individuals must act by predicting the risk of falls caused by declines in sensory function. Thus, we propose that prior knowledge of a disturbance helps compensate deteriorating sensory functions in older subjects and hypothesize that balancing ability may change if predictive factors are activated by supplying information on the timing of a perturbation in advance. This hypothesis is supported by Shinya et al, [26]. That described that one of the important issues in the function of the central nervous system associated with postural control was “to cope with the uncertainty and unpredictability of real-world perturbations.” They examined posture control upon perturbation of the supporting surface using a splitbelt treadmill and investigated how prior knowledge of perturbation affects latent muscle reflex. Reflexive muscle activity is a part of the automatic postural response and is a complex and sophisticated pattern of muscle activity to cope with various disturbance stimuli. Thus, the activity of the gastrocnemius and soleus muscles of the right lower limb decreased and that of the tibialis anterior muscle increased when the prediction of acceleration was provided. Moreover, latency activity shortened and reaction after a disturbance was faster when prior knowledge of the timing of acceleration and deceleration was provided. These results suggest that prior knowledge may enhance the posture control system like balancing ability through the activity of the lower thigh muscles. However, while many reports have examined muscle activity and evaluated behavior with or without prior knowledge, there have been no reports on the association between neural activity of the brain regions and predictive factors such as self-efficacy or actual balancing ability when a postural perturbation is applied.
In this study, we aim to examine how the body’s balancing ability and fall-related self-efficacy in older subjects are related to the brain regions that may control predictive factors during a postural perturbation, and how prior knowledge in advance of a postural perturbation affects these relationships. The present study is the first to examine whether prior knowledge has a compensatory effect on the brain functions that create predictive factors in older individuals. Clarifying these relationships may help to create an approach for fall prevention.
Materials and Methods
Study participants
During the convenience sampling process, we recruited 30 older men living in the community. Those with orthopedic, neurological, mental, movement, or sensory disorders were excluded from the study. Those who had experienced falls were also excluded because it affects fall-related self-efficacy [27]. As a result, 17older men (average age of 76.4 ± 5.8 years) were included in the study (Table 1). The methodology and purpose of this study were explained to the subjects, both in writing and verbally, prior to obtaining their written consent to participate. This study was approved by the Kyoto Tachibana University (approval number: 19-08). Owing to its Crosssectional study, this study was conducted in line with the STROBE guidelines.
Factor name
n = 17
Sex
Male (n = 17)
Age
76.4 (±5.8)
Height
163.6 (±7.5)
Weight
58.0 (±8.9)
Self-efficacy score of falls
116.7 (±27.6)
Mini-BESTest score
Anticipatory
4.8 (±0.9)
Postural responses
4.6 (±0.6)
Sensory orientation
5.8 (±0.6)
Dynamic gait
8.6 (±1.6)
Total
23.8 (±2.5)
Abbreviations: Mini-BESTest: Mini-Balance Evaluation Systems Test.
Table 1: Subject attributes.
Experimental Environment
The test floor used to induce the postural perturbations was prepared by fixing a wheeled trolley, the equipment made of an iron plate with dimensions of 105 × 105 cm, with pillars on its sides and back (Figure 1). The translational motion (postural perturbations) of the test floor was a rapid rocking to the right by an actuator. Physical movement was detected using a fixed motion-activated camera (NaturalPoint, Inc. Prime13W, USA). At the time of measurement, subjects stood on the test floor with an electroencephalogram to evaluate brain function with a reflective marker of the 3D motion analysis device attached. In addition, safety belts fixed with pillars were attached to the trunk of the subjects to eliminate the risk of falling. The safety belt did not prevent any body movements.
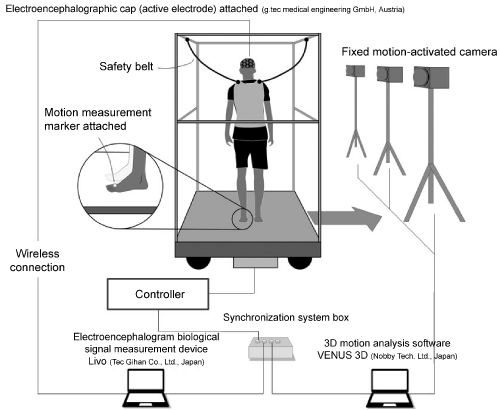
Figure 1: Experimental environment and measurement outline.
Procedure
Dynamic balancing ability and fall-related self-efficacy of each subject were evaluated prior to measurements. Then, an electroencephalogram device, which evaluated brain function, and a reflex marker, which evaluated body function during postural perturbations, was attached to the subject. Subsequently, in the patients given or not given prior information about the timing of the postural perturbations, three phases were measured: 1) Before, 2) during (lateral perturbation), and 3) after the postural perturbations. Once the subjects boarded the trolley, they were instructed to position their feet in a resting posture. Subjects were instructed to remain silent during the experiment to control speech and unnecessary movements, as these can affect measurements of electroencephalograms and physical function. To measure the control of visual information under these conditions, subjects were instructed to look at a marking tape positioned at eye-level 2 m in front of their eyes.
Under the “prior knowledge” condition, the timing of movement of the trolley was given to the subjects in advance and the movement of the trolley was announced in advance. In contrast, under the “without prior knowledge” condition, no prior information was given to the subjects. Under “prior knowledge” settings, subjects were given the instruction “The floor moves at the signal of 2, 1, 0 (timing of 0)” and “Please prepare,” followed by “2, 1, 0.” Thereafter, the floor surface was moved beginning at “0.” Under “without prior knowledge” condition, subjects were only told “Please stand still” as a signal that began the experiment, and then the trolley was moved. To prevent subjects from predicting the time taken to move the trolley, its timing was set at 3,000 ± 500 ms after the last instruction was given. For every measured phase, the “before the postural perturbations” phase evaluated the brain function activity related to outcome expectation, showing the state of movement preparation that reflects the motor image in the brain. Since the electric potential of preparation activity was reflected 2 seconds before the movement [27], this phase was set at 3 seconds from the signal time. To maintain consistency throughout the experiment, “during the postural perturbations” and “after the postural perturbations” phases were also set to 3 seconds. For the 3-second measurements, EEG data were used for the 2-second period before the end trigger before the postural perturbations, and for the 2-second period after the start trigger during and after the postural perturbations.
In this experiment, a total of six measurements were made per subject; a measurement was made at each of the three phases in one task, and three measurements were performed under each condition. A sufficient interval was provided between each measurement. Moreover, the order of the conditions was changed for each subject, to prevent it from affecting the results.
Evaluation of Balancing Ability
The dynamic balancing ability was evaluated prior to the experiment using The Mini-Balance Evaluation Systems Test (Mini- BESTest) [28]. The Mini-BESTest was developed from 14 tasks in four sections (e.g., anticipatory, postural responses, sensory orientation, and dynamic gait) required to evaluate dynamic balance function by factor analysis and Rasch analysis. These include 36 tasks from the six sections related to balance function. The high reliability and validity of the Mini-BESTest have been reported by Godi et al, [29]. The present study used the Japanese version of the Mini-BESTest (J-Mini- BESTest).
Fall Self-Efficacy
Fall self-efficacy was evaluated using the Japanese version of the Falls Efficacy Scale (JFES) developed by Maeba and [30], based on the Falls Efficacy Scale by Tinetti et al, [11] described previously. The self- confidence of performing each of the 15 activities involved in daily life without falling was evaluated on a scale of 10 (1: not confident, 10: extremely confident), and a high total score indicated a high fallrelated self-efficacy.
Evaluation of Brain Function
Electroencephalogram measurements were performed to evaluate brain function. Each measurement was recorded using g. SAHARA’s active electrodes (G.tec Medical Engineering GmbH, Austria) and a biological signal measuring device Livo (Tec Gihan Co., Ltd., Japan). Based on the International 10–20 system, the measurements were taken from 15 sites: Fpz, Fz, Cz, Pz, Oz, F3, F4, C3, C4, P3, P4, F7, F8, T7, and T8, and using a bandpass filter of 1–30 Hz to remove artifacts during postural perturbations with a sampling frequency of 1,000 Hz. In this study, the electroencephalogram data of one task were collected for 2 seconds and analyzed for each phase. Standardized Low-Resolution Brain Electromagnetic Tomography (sLORETA), a three-dimensional functional brain imaging filter developed by [31], was used for the analysis. The electroencephalogram data was standardized using the Talairach Daemon software [32] incorporated in the analysis program. Subsequently, the data was calculated as the coordinate values of the Montreal Neurological Institute (MNI) standard brain coordinate system in the x, y, and z axes in the brain regions divided into 6,239 voxels using sLORETA analysis and converted into a three-dimensional image. As a result, the neural activity value for each task condition was calculated as the current density value (μA/mm2) on each voxel. After calculating the neural activity in each of the three phases under each condition, we used this method to calculate the neural activity value in each brain coordinate region that showed the highest neural activity value in the SMA and right IPL regions in the three phases under the two conditions. Since the SMA acts synchronously on both sides of the brain when creating a motor image [33], that of the right hemisphere, which is more involved in the representation of one’s own body in the brain [34], was used in this study. To determine the brain functional activity specialized in the postural perturbations, the data obtained by subtracting the electroencephalogram measurement during the standing motion measured in advance was used. We also analyzed neural connections to examine whether these regions work cooperatively in motor control during postural perturbations. The lagged linear connectivity analysis incorporated into sLORETA, which uses the Hermitian covariance matrices, was used to analyze neural connectivity [35]. We compared neural connections of both brain regions with and without prior knowledge. Linear wires (red wires for positive correlation and blue wires for negative correlation) were drawn in the brain imaging provided a strong neural correlation was observed at the time of postural perturbation (statistical significance level of 5%).
Evaluation of Physical Functions
We measured the time at which the motor reaction of the foot occurred after the postural perturbation stimulation (foot response time). Foot response time was used as an index to examine the relationship between neural activity in the brain and the timing of muscle contraction activation excluding the peripheral mechanism [36]. Moreover, the motor reaction time of the left foot was measured in this experiment since muscle groups that control the movements of the ankle joints, such as the tibialis anterior, peroneus longus, and soleus, are active at the initial stage of a lateral disturbance of the floor [37,38]. Foot motion response during a postural perturbation was measured by an OptiTrack motion capture system (NaturalPoint, Inc. USA) using a fixed motion-activated camera. The data were analyzed using the 3D motion analysis software VENUS 3D (Nobby Tech. Ltd., Japan). Reflective markers were placed on the upper surface of the trolley at the knee joint point, the malleolus and the apex (metacarpophalangeal joints of the second toe), and the reference point. We measured the time (ms) from the average position of the reference point marker within 500 ms of the trolley start signal, up to the time point that indicated maximum amount of upward movement of the left foot apex point (the base of the second finger) after the start of the trolley (at the time when the distance between the reference point and the foot apex point was at the maximum). Sampling frequency was 200 Hz. Calculated foot response time was converted into a natural logarithm (log) to approximate a normal distribution and use as a foot response value.
Statistical Analysis
The relationship between the J-Mini-BESTest scores, the JFES scores, SMA and the right IPL neural activity values, and foot response values was analyzed under each tested condition. The normality test for each data group was performed using the Kolmogorov-Smirnov test. Statistical processing for the analysis was performed using the Pearson product-moment correlation coefficient. A test of no correlation was performed to determine whether the observed effects were caused by a bias in the sample size. Furthermore, we performed a simple regression analysis to verify the effects of neuronal activity on balancing ability and fall-related self-efficacy in the items with correlation. SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis, and the two-sided significance level was set at 0.05.
Results
Table 2 shows the results of the J-Mini-BESTest scores, the JFES scores, and current density and foot response values of the SMA and right IPL for each phase, with and without prior knowledge of the timing of a perturbation. The normality test showed normal distribution of the data.
Phase
Before postural perturbations
During postural perturbations
After postural perturbations
Before postural perturbations
During postural perturbations
After postural perturbations
Condition
Region of Interest
SMA MNI coordinates (x, y, z) (6, -4, 55)
IPL MNI coordinates (x, y, z) (50, -64, 25)
With prior knowledge
Current density value (μA/mm2)
Current density value (μA/mm2)
Foot response value (log)
0.43±0.09
0.44±0.11
0.45±0.09
0.41±0.06
0.46±0.05
0.43±0.06
6.5±0.3
Without prior knowledge
0.39±0.11
0.42±0.07
0.47±0.09
0.38±0.05
0.45±0.06
0.41±0.06
6.7±0.5
Abbreviations: SMA: Supplementary Motor Area; IPL: Inferior Parietal Lobule; MNI: Montreal Neurological Institute
Table 2: Neural activity value and foot response value in each phase with or without prior knowledge.
A significant positive correlation was found between the J-Mini- BESTest and the JFES scores (r = 0.68, p = 0.002) (Table 3). We examined the correlation of self-efficacy on balancing ability, which is a factor for falls in the elderly assessed in both scores. A significant association between the JFES and the J-Mini-BESTest scores was observed on simple regression analysis using the J-Mini-BESTest scores as the dependent variable and JFES scores as the independent variable (R² = 0.47, p < 0.01) (Figure 2a). With prior knowledge of the timing of a perturbation, the J-Mini-BESTest scores showed a significant positive correlation with neural activity values (r = 0.51, p = 0.035) of the SMA before a postural perturbation (MNI coordinate: 6,-4,55) (Table 3). A simple regression analysis was performed to determine whether the SMA neural activity inducing motor imagery affects balancing ability. SMA neural activity values before postural perturbation were significantly associated with the J-Mini-BESTest scores, as shown by the simple regression analysis using the J-Mini- BESTest scores as the dependent variable and SMA neural activity values before postural perturbation as independent variable (R2 = 0.26, p < 0.05) (Figure 2b).
With prior knowledge
JFES score
J-Mini-BESTest score
Foot response value
Before postural perturbations - SMA nerve activity value(μA/mm2)
After postural perturbations - IPL nerve activity value(μA/mm2)
JFES score
-
0.68*
-0.19
0.35
0.52*
J-Mini-BESTest score
-
-0.40
0.51*
0.56*
Foot response value
-
-0.69*
-0.03
Before postural perturbations - SMA nerve activity value
-
0.39
After postural perturbations - IPL nerve activity value
-
Spearman's rank correlation coefficient*; p<0.05
Abbreviations: J-Mini-BESTest Score: Japanese Version of the Mini-BESTest; JFES: Japanese Version of Falls Efficacy Scale; SMA: Supplementary Motor Area; IPL: Inferior parietal Lobule
Table 3: JFES scores, J-Mini-BESTest scores, correlation of neural activity values at SMA and right IPL, and partial correlation coefficient.
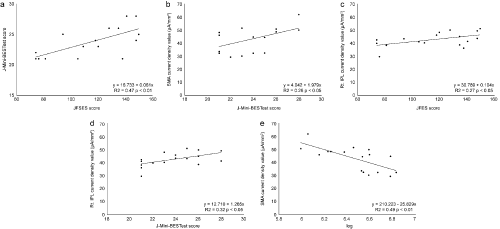
Figure 2: Association between J-Mini-BESTest scores, JFES scores, and neural activity (regression line). a) Association between J-Mini-BESTest scores and
JFES scores. JFES scores were significantly associated with J-Mini-BESTest scores (R2 = 0.47, p < 0.01). b) Association between SMA neural activity and J-Mini-
BESTest scores before a postural perturbation. SMA neural activity values before a postural perturbation were significantly associated with J-Mini-BESTest scores
(R2 = 0.26, p < 0.05). c) Association between the right IPL neural activity and JFES scores after a postural perturbation. Right IPL neural activity values after a
postural perturbation were significantly associated with JFES scores (R2 = 0.27, p < 0.05). d) Association between the right IPL neural activity and J-Mini-BESTest
scores after a postural perturbation. Right IPL neural activity values after a postural perturbation were significantly associated with J-Mini-BESTest scores (R2 =
0.32, p < 0.05). e) Association between neural activity values and foot reaction values (log) of SMA before a postural perturbation. SMA neural activity values before
a postural perturbation were significantly associated with foot response values (R2 = 0.49, p < 0.01).
Abbreviations: J-Mini-BESTest: Japanese Version of Mini-Balance Evaluation Systems Test; JFES: Japanese Version of the Falls Efficacy Scale; SMA:
Supplementary Motor Area; IPL: Inferior Parietal Lobule.
In addition, the neural activity values of the right IPL after a postural perturbation (MNI coordinate: 50, -64, 25) showed a significant positive correlation with JFES (r = 0.52, p = 0.032) and J-Mini-BESTest scores (r = 0.56, p = 0.019) (Table 3). A significant negative correlation was observed between foot response values and neural activity values of the SMA before a postural perturbation (r = -0.69, p = 0.002) (Table 3). A simple regression analysis was performed to determine whether the neural activity of the right IPL, inducing a sense of agency, affected fall-related self-efficacy or postural control. The results showed a significant association between the neural activity of the right IPL after a postural perturbation and the JFES scores, when the JFES scores were used as the dependent variable and the right IPL neural activity values after a postural perturbation as independent variable (R² = 0.27, p < 0.05) (Figure 2c). Furthermore, a significant association between the neural activity values of the right IPL after a postural perturbation and J-Mini-BESTest scores was observed by simple regression analysis using J-Mini-BESTest scores as the dependent variable and the right IPL neural activity values after postural perturbation as the independent variable (R² = 0.32, p < 0.05) (Figure 2d). In addition, simple regression analysis was performed to determine whether the neural activity of SMA prior to a disturbance influences physical responses. The neural activity values of the SMA before a postural perturbation were significantly related to the foot response values (R² = 0.49, p < 0.01) as shown by simple linear regression analysis using foot response values as dependent variable and the neural activity values of the SMA before a postural perturbation as independent variable (Figure 2e). Furthermore, simple regression analysis revealed that the significant correlation coefficients among the independent variables were all moderate or lower, and there was no problem of multi-collinearity. The results of the uncorrelated tests for significant correlation coefficients were all significant.
Finally, during postural perturbation, a significant positive neural correlation between the SMA and right IPL during motor control was observed in the neural activity in both brain regions under conditions of prior knowledge of postural perturbation (Figure 3).
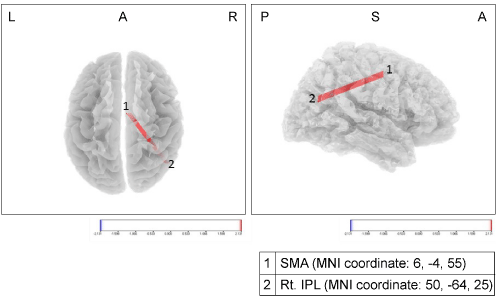
Figure 3: Comparison of neural connection between the conditions with and without prior knowledge. Left panel, view from above the horizontal plane. (L, Left; A,
Anterior; R, Right). Right panel, view from the right side of the sagittal plane. (P, Posterior; S, Superior; A, Anterior). Under conditions of prior knowledge, there was
a strong significant positive correlation (red wire) between the neural activities of the SMA and right IPL during a postural perturbation, as compared to that with
conditions of no prior knowledge. The numerical values of the color scale below the image represent the t values (t = 2.131).
Abbreviations: SMA: Supplementary Motor Area; IPL: Inferior Parietal Lobule; MNI: Montreal Neurological Institute.
Discussion
Prevention of falls in older individuals requires an improvement in their ability to balance, and high self-efficacy against falls can be maintained intentionally. In this study, we hypothesized that selfefficacy was a crucial basic predictive factor that regulates movements and behavior of individuals. Thus, we examined its relationship with the balancing ability, and the relationship between the functional areas of the brain that are the basis of physical movement functions (e.g., motor image) and a sense of agency. Furthermore, we believe that elucidating how prior knowledge affects compensatory brain functions to create a predictive factor in older individuals with impaired sensory function could help the development of an approach to prevent falls caused by balance disorders.
The biggest risk factor for falls in older individuals is lower limb weakness [38]. However, since several factors involved in the falls of older individuals are complex, their falls are thought to involve not only physical characteristics but also superior function factors, such as fear of falling and deteriorated attentional functions. Moreover, psychological effects after a fall in this population are expected to have a significant impact on their prognosis, including reduced levels of autonomous daily living ability and limited social participation. In this study, we observed a significant positive correlation between balancing ability and fall-related self-efficacy in older individuals; fallrelated self-efficacy was found to affect balancing ability. In addition, with prior knowledge of the timing of a postural perturbation, balancing ability scores and fall-related self-efficacy scores had a significant positive correlation with neural activity values of the right parietal lobe after postural perturbation. [27] examined the relationship between physical activity patterns, self-efficacy, balancing ability, and fear of falling in older individuals. By comparing inactive older individuals, such as institutional residents, with active older individuals, the authors found that active older individuals had less fear of falling, a better balance, and higher self-efficacy. They also reported that those with high balancing ability had less fear of falling, and although balancing ability and self-efficacy acted as significant independent variables in the fear of falling, the degree of physical activity was not a significant variable. Moreover, [39] examined the relationship between four variables in older individuals: fear of falling, fall-related self-efficacy, fall experience, and physical function. Fallrelated self-efficacy was shown to be the greatest variable predicting current physical function. Thus, our findings on the relationship between balancing ability and fall-related self-efficacy in this study support these reports.
Balancing ability and fall-related self-efficacy were found to be associated with each other and showed an association with neural activity values in the right parietal lobe after a postural perturbation. The right IPL is associated with the development of a sense of agency, which includes self-efficacy [40]. A retrospective process (apparent mental causation), which is generated by reasoning performed by recalling and associating experiences after carrying out movements, is important to establish a sense of agency necessary for motor control [41]. Furthermore, with prior knowledge of the timing of a postural perturbation, subjects were able to clearly predict movement results prior to the actual movements. Thus, the sense of agency was further enhanced since the actual results and predictions were likely to match and the predictive process (comparator model) was executed, which is also necessary for the establishment of the sense of movement [42]. Simple regression analysis revealed that neural activity in the right IPL may affect fall-related self-efficacy. This result supports Moore’s [21] idea that the sense of agency is caused by the integration of the information stored in the brain (internal cue) and the detailed information that arrives externally (external cue). In contrast, regarding results showing the relationship between balancing ability and parietal lobe activity, it has been reported that spatial localization ability was transiently reduced when the function was temporarily suppressed by repeated transcranial magnetic stimulation to the right parietal association area [43]. Thus, the right IPL is presumed to process sensory information necessary for adjustments of body balance. Moreover, the IPL is a brain region associated with motor learning (a sequence late in the learning process) [44], and the right IPL in particular is involved in the processing of feedback from visual and verbal cues [45]. In this study, we observed that balancing ability positively correlated with neural activity values of the SMA before a postural perturbation. Thus, we think this may be because individuals with a higher balancing ability had a more detailed predictive image of movements via prior knowledge and its association with the neural activity reflects motor learning that occurred after the postural perturbation.
We also observed that having prior knowledge of the timing of a postural perturbation resulted in the neural activity values of the SMA before a postural perturbation showing significant correlation with balancing ability scores and foot response values. When an individual obtains prior information about an external stimulus that induces a fall, the individual prepares for stimulation in the brain and collects information from input systems, such as the visual, vestibular, somatosensory, and auditory systems. In contrast, the movement program corresponding to the stimuli is planned by integrating the two while recalling the past motor memory information to respond to external stimuli [46]. Movement prediction based on these is activated in response to the postural perturbations, which proactively contracts muscles involved in posture control and selects the cocontraction system of muscle groups required for movements. This may predict the deviation of the body’s center of gravity and control body posture [47], and the mechanism that predicts the deviation of the body’s center of gravity and controls posture in movement preparation is called anticipatory postural adjustments [48], which is carried out partly by the SMA [49]. In general, the motor region and motor cortex region, including the SMA, are connected to the basal ganglia and cerebellum, forming a motor loop that contributes to the execution of voluntary movements and motor programming [50]. Among these, the SMA contributes to the predictive adjustment of footstep control [22], transfers accurate foot movement programs to the motor cortex (M1), and sends movement commands via the corticospinal tract [51]. Furthermore, it is involved in the construction of motor components (e.g., motor programs) based on internal memory in the brain. Moreover, this region exhibits high neural activity upon actual movements, but becomes active simply by imagining movements in the brain even without actual movement [52], and high motor image ability is indicated by the high neural activity of the SMA [53]. This indicates that the SMA plays a crucial role in the generation and storage of movement programs in the preparatory stage of movements. Since the dysfunction of this region interferes with postural control without the induction of motor function paralysis, the SMA is speculated to be associated with predictive postural adjustment. Based on these, since the SMA of individuals with higher balancing ability likely has more motor memory information patterns, it is possible that an individual with a higher balancing ability can induce a more detailed movement-image predictively by obtaining prior knowledge. Thus, the association between the two was observed in this study. In addition, this study showed the association between neural activity of the SMA and foot response values. A study investigated the effect of prior knowledge on muscle responses of the lower limbs in response to perturbations during walking [54]. The authors found that the latency of the muscle activity response of the tibialis anterior muscle was shortened with prior knowledge of perturbation, showing a faster response, than that without prior knowledge. This suggests that prior knowledge (e.g., recognition of perturbation) contributes to posture control through appropriate muscle contraction activity [53]. These studies clearly indicate that the postural control system of the central nervous system regulates movement strategy and muscle contractile activity, utilizing prior knowledge of potential future postural perturbations. Therefore, this study suggests an association between neural activity of the SMA and the reaction time of the foot.
A significant positive correlation between the neural activities of the SMA and right IPL during motor control was observed during postural perturbations when prior information was given. In contrast, the correlation was not observed without prior knowledge of a postural perturbation. It has been reported that the IPL causes a strong desire for body movement and motor intention with the neural activity [55], and this region is believed to work predominantly, especially during execution of the first-person image. For postural perturbations, it is important to predict the input sensory information, simulating this as movement-image prior to the corresponding physical movement. The SMA and IPL, in which the neural connection was observed in this study, are considered to be the central regions responsible for the simulation function [56]. In intrinsic movement planning, such as movement-image, the exchange of neural information preceded by the IPL, which predicts movement results, is made in all brain regions, including the SMA [57]. Prior knowledge has a large compensatory effect on the prediction of movement, demonstrating the functional association between the two brain regions when prior information was given. The IPL is associated with the induction of the sense of agency. In the experience of the sense of agency, not only perceptual but also cognitive stimulus elements are important, and it has been suggested that its development influences motor control [58]. Moreover, it has been shown that SMA neural activity increases, in contrast to IPL activity, when prediction error is large, and a sense of agency is less likely to develop [57]. Based on this information, both brain regions are considered to act cooperatively, since prior knowledge becomes a compensatory factor to counteract the decline of sensory input function.
Several traditional interventions on fall prevention have focused on a single factor, such as improvement of the motor function of an individual [59]. Postulated a conceptual model of aging, control, and motivation, and proposed a multifactorial intervention strategy based on this model. In recent years, such approaches have been incorporated into fall prevention interventions with considerably positive effects [60]. Therefore, future fall prevention programs will require a multifactorial approach that includes both physical functions and fall-related self-efficacy. When an individual makes a movement, it is necessary to select the sensory information associated with the actions in an environment. For this, the credibility of judgment is weighted according to the amount of information on the event, and it is difficult for older individuals to connect a relatively uncertain event to a more reliable one under an information overload. Therefore, we believe that the consistency between actions and results can be maintained by establishing predictive factors through prior knowledge.
Limitations
Our study has several limitations. First, although we suggested that fall-related self-efficacy may affect balancing ability, a causal relationship could not be established due to the cross-sectional design of this study [61]. Suggested that self-efficacy could be the main factor affecting the behavior of an individual when the individual has sufficient ability to act and use skills to achieve a special goal. Therefore, the two may have a mutual relationship, rather than a unidirectional relationship [62], and these may need to be prospectively examined using tracking survey data derived from this study. Second, in examining the neural basis of factors affecting mental and physical functions, such as balance and self-efficacy, we focused on the neural activity of SMA and IPL and compared them. These regions are undoubtedly central to the generation of motor image and sense of subjectivity, but it has also been shown that they are based on the formation of networks with other regions of the cerebrum and the cerebellum. In the future, it is necessary to analyze the network in detail from both a spatial and a temporal perspective. Third, since this study used balance and mental state of older individuals as assessment data, individual and background factors may have had a significant effect on the results. However, as this study was not an inter-individual comparison experiment, our results may not be affected by individual and background factors. Finally, due to the limited sample size, our study results cannot be generalized. It is therefore warranted to compare our results with those of other studies that used a larger sample size.
Conclusion
This study showed a significant association between balancing ability and fall-related self-efficacy. With prior knowledge of a postural perturbations, both balancing ability and fall-related selfefficacy showed a significant association with the neural activity of the right IPL after a postural perturbation. In addition, the neural activity of the SMA before a postural perturbation was significantly associated with balancing ability and foot response. Furthermore, we found that the brain region responsible for the predictive factor during a postural perturbation may enhance functional connection with prior knowledge. These results showed that prior knowledge may be a factor that connects the activity of neurological functioning, which is the basis of the predictive factor, and balancing ability. Therefore, to prevent falls, older individuals should increase all parameters of the predictive factor by understanding their environment and physical condition in advance. Since older individuals vary widely in terms of their individual and environmental factors, it is necessary to include a broader range of older adults in future studies to generalize the present study’s results.
Acknowledgments
We would like to thank all the volunteers who participated in this study.
Funding
This study was supported by JSPS KAKENHI Grant Number 22K19696.
Declarations of Interest
The authors have no conflict of interests to declare.
Author Contributions
Study conceptualization, T.K.; Methodology, T.K. and H.Y.; Investigation, T.K., T.A., N.M., and H.Y.; Writing-original draft, T.K.; Writing-review and editing, T.K., T.A., N.M., and H.Y.; Resources, H.Y.; Supervision, T.A. and H.Y.
Highlights
• Falls in older individuals can be caused by balance disorders
• We demonstrate association between balancing ability and fallrelated self-efficacy
• Prior postural perturbations knowledge associated with right IPL neural activity
• SMA and right IPL neural activity positively correlated during perturbation
References
- Wang D, Zhang J, Sun Y, Zhu W, Tian S, Liu Y. Evaluating the fall risk among elderly population by choice step reaction test. Clinical Interventions in Aging. 2016; 2016: 1075-1082.
- Tuerk C, Zhang H, Sachdev P, Lord SR, Brodaty H, Wen W, et al. Regional Gray Matter Volumes Are Related to Concern About Falling in Older People: A Voxel-Based Morphometric Study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2016; 71: 138-144.
- Uusi-Rasi K, Karinkanta S, Tokola K, Kannus P, Sievänen H. Bone Mass and Strength and Fall-Related Fractures in Older Age. Journal of Osteoporosis. 2019; 2019: 1-6.
- Wu S, Rau C, Kuo SCH, Chien P, Hsieh C. The influence of ageing on the incidence and site of trauma femoral fractures: a cross-sectional analysis. BMC Musculoskeletal Disorders. 2019; 20.
- Higashikawa T, Shigemoto K, Goshima K, Usuda D, Okuro M, Moriyama M, et al. Risk factors for the development of aspiration pneumonia in elderly patients with femoral neck and trochanteric fractures. Medicine. 2020; 99: e19108.
- Moore DS, Ellis R. Measurement of fall-related psychological constructs among independent-living older adults: A review of the research literature. Aging & Mental Health. 2008; 12: 684-699.
- Rinkel WD, Nieuwkasteele SV, Cabezas MC, Neck JWV, Birnie E, Coert JH. Balance, risk of falls, risk factors and fall-related costs in individuals with diabetes. Diabetes research and clinical practice. 2019; 158: 107930.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological review. 1977; 84: 191-215.
- Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall. 1986.
- Bandura A. Self-efficacy: The exercise of control. W.H Freeman and Company. 1997.
- Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. Journal of gerontology. 1990; 45: P239-P243.
- Dewi SR. Self Efficacy, Postural Balance and Fall Risk on Elderly in Upt Pstw Jember. Procceeding the 4th International Nursing Conference. 2019.
- Huxham FE, Goldie PA, Patla AE. Theoretical considerations in balance assessment. The Australian journal of physiotherapy. 2001; 47: 89-100.
- Naito E, Kochiyama T, Kitada R, Nakamura S, Matsumura M, Yonekura Y, et al. Internally Simulated Movement Sensations during Motor Imagery Activate Cortical Motor Areas and the Cerebellum. The Journal of Neuroscience. 2002; 22: 3683-3691.
- Simone LD, Tomasino B, Marusic N, Eleopra R, Rumiati RI. The effects of healthy aging on mental imagery as revealed by egocentric and allocentric mental spatial transformations. Acta psychologica. 2013; 143: 146-156.
- Saimpont A, Malouin F, Tousignant B, Jackson PL. Motor Imagery and Aging. Journal of Motor Behavior. 2013; 45: 21-28.
- Personnier P, Ballay Y, Papaxanthis C. Mentally represented motor actions in normal aging: III. Electromyographic features of imagined arm movements. Behavioural Brain Research. 2010; 206: 184-191.
- Blakemore S, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends in Cognitive Sciences. 2002; 6: 237-242.
- Rochat P, Morgan R. Spatial determinants in the perception of self-produced leg movements in 3- to 5-month-old infants. Developmental Psychology. 1995; 31: 626-636.
- Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences. 2000; 4: 14-21.
- Moore J W. What is the sense of agency and why does it matter? Frontiers in Psychology. 2016; 7: 1272.
- Kodama T, Nakano H, Katayama O, Murata S. The association between brain activity and motor imagery during motor illusion induction by vibratory stimulation. Restorative Neurology and Neuroscience. 2017; 35: 683-692.
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. NeuroImage. 2003; 18: 324-333.
- Yomogida Y, Sugiura M, Sassa Y, Wakusawa K, Sekiguchi A, Fukushima A, et al. The neural basis of agency: An fMRI study. NeuroImage. 2010; 50: 198-207.
- Silva LAD, Jaluul O, Teixeira MJ, Siqueira JTTD, Filho WJ, Siqueira SRDTD. Quantitative sensory testing in elderly: longitudinal study. Arquivos de neuropsiquiatria. 2018; 76: 743-750.
- Shinya M, Kawashima N, Nakazawa K. Temporal, but not Directional, Prior Knowledge Shortens Muscle Reflex Latency in Response to Sudden Transition of Support Surface During Walking. Frontiers in Human Neuroscience. 2016; 10.
- McAuley E, Mihalko S L, Rosengren K. Self-efficacy and balance correlates of fear of falling in the elderly. Journal of Aging and Physical Activity. 1997; 5: 329-340.
- Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini- BESTest. Journal of rehabilitation medicine. 2010; 42: 323-331.
- Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of Reliability, Validity, and Responsiveness of the Mini-BESTest and Berg Balance Scale in Patients With Balance Disorders. Physical Therapy. 2012; 93: 158-167.
- Maeba K, Takenaka K. [Factors affecting falls self-efficacy of home-bound elderly people]. Nihon Ronen Igakkai zasshi. Japanese journal of geriatrics. 2010; 47: 323-328.
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods and findings in experimental and clinical pharmacology. 2002; 24: 5-12.
- Talairach J, Teurnoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers Inc. 1988: 132.
- Kodama T, Katayama O, Nakano H, Ueda T, Murata S. Treatment of Medial Medullary Infarction Using a Novel iNems Training: A Case Report and Literature Review. Clinical EEG and Neuroscience. 2019; 50: 429-435.
- Daprati E, Sirigu A, Nico D. Body and movement: Consciousness in the parietal lobes. Neuropsychologia. 2010; 48: 756-762.
- Pascual-Marqui R D. Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: Frequency decomposition. Arxiv preprint. 2007. arXi: 07111455.
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. Journal of neurophysiology. 1998; 80: 1939-1950.
- Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. Journal of neurophysiology. 2004; 91: 489-501.
- Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle Weakness and Falls in Older Adults: A Systematic Review and Meta-Analysis. Journal of the American Geriatrics Society. 2004; 52: 1121-1129.
- Tinetti ME, Leon CFMD, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. Journal of gerontology. 1994; 49: M140-M147.
- Ohata R, Asai T, Kadota H, Shigemasu H, Ogawa K, Imamizu H. Sense of Agency Beyond Sensorimotor Process: Decoding Self-Other Action Attribution in the Human Brain. Cerebral Cortex (New York, NY). 2020; 30: 4076-4091.
- Wolpe N, Rowe JB. Beyond the “urge to move”: objective measures for the study of agency in the post-Libet era. Frontiers in Human Neuroscience. 2014; 8.
- Haggard P. Conscious intention and motor cognition. Trends in Cognitive Sciences. 2005; 9: 290-295.
- Xu G, Lan Y, Zhang Q, Liu D, He X, Lin T. 1-Hz Repetitive Transcranial Magnetic Stimulation over the Posterior Parietal Cortex Modulates Spatial Attention. Frontiers in Human Neuroscience. 2016; 10.
- Lafleur MF, Jackson PL, Malouin F, Richards CL, Evans AC, Doyon J. Motor Learning Produces Parallel Dynamic Functional Changes during the Execution and Imagination of Sequential Foot Movements. NeuroImage. 2002; 16: 142-157.
- Halsband U, Lange RK. Motor learning in man: A review of functional and clinical studies. Journal of Physiology-Paris. 2006; 99: 414-424.
- Anokhin PK. Biology and neurophysiology of the conditioned reflex and is role in adaptive behavior. Pergamon Press Ltd. 1974; 3.
- Takakusaki K. Functional Neuroanatomy for Posture and Gait Control. Journal of Movement Disorders. 2017; 10: 1-17.
- Massion J. Movement, posture and equilibrium: Interaction and coordination. Progress in Neurobiology. 1992; 38: 35-56.
- Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience. 2009; 164: 877-885.
- Hikosaka O. GABAergic output of the basal ganglia. Progress in brain research. 2007; 160: 209-226.
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Current Opinion in Neurobiology. 2007; 17: 234-242.
- Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, et al. Dominance of the right hemisphere and role of area 2 in human kinesthesia. Journal of neurophysiology. 2005; 93: 1020-1034.
- Heiden TL, Sanderson DJ, Inglis JT, Siegmund GP. Adaptations to normal human gait on potentially slippery surfaces: the effects of awareness and prior slip experience. Gait & posture. 2006; 24: 237-246.
- Shinya M, Oda S. Fast muscle responses to an unexpected foot-inhole scenario, evoked in the context of prior knowledge of the potential perturbation. Experimental Brain Research. 2010; 203: 437-446.
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement Intention After Parietal Cortex Stimulation in Humans. Science. 2009; 324: 811-813.
- Gerardin E, Sirigu A, Lehéricy S, Poline JB, Gaymard B, Marsault C, et al. Partially overlapping neural networks for real and imagined hand movements. Cerebral cortex. 2000; 10: 1093-1104.
- Sperduti M, Delaveau P, Fossati P, Nadel J. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Structure and Function. 2010; 216: 151-157.
- Gentsch A, Synofzik M. Affective coding: the emotional dimension of agency. Frontiers in Human Neuroscience. 2014; 8.
- Lachman M E, Jette A, Tennstedt S, Howland J, Harris B A, et al. A cognitivebehavioral model for promoting regular physical activity in older adults. Psychology, Health & Medicine. 1997; 2: 251-261.
- Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The Effectiveness of a Community-Based Program for Reducing the Incidence of Falls in the Elderly: A Randomized Trial. Journal of the American Geriatrics Society. 2004; 52: 1487-1494.
- Feltz D L, Payment C A. Self-efficacy beliefs related to movement and mobility. Quest, 2005; 57: 24-36.
- Rochat S, Büla CJ, Martin E, Seematter-Bagnoud L, Karmaniola A, Aminian K, et al. What is the relationship between fear of falling and gait in wellfunctioning older persons aged 65 to 70 years?. Archives of physical medicine and rehabilitation. 2010; 91: 879-884.