
Research Article
Phys Med Rehabil Int. 2022; 9(3): 1206.
Decannulation Timing is an Expression of Neurological Recovery Time: The Need to Define a Performance within a Time Frame
Lombardi F*, Briganti A, Colli G, Marchetti P, Massobrio M and Lusuardi M
Department of Neuromotor Rehabilitation, Azienda USLIRCCS di Reggio Emilia, Italy
*Corresponding author: Lombardi F, Department of Neuromotor Rehabilitation, Azienda USL-IRCCS di Reggio Emilia, Via Mandriolo Superiore 15, 42015 Correggio, Italy
Received: October 03, 2022; Accepted: October 31, 2022; Published: November 07, 2022
Abstract
Introduction: The factors that influence the likelihood of tracheostomy tube weaning in patients with severe acquired brain injury (sABI) are fairly well known, and recently it has been pointed out that improving swallowing and cough resistance are among the most important. What has not yet been focused on is the “passage of time” factor and how much it affects weaning opportunities.
Objective: With this study, we wanted to analyze how the probability of decannulation varies over time, consistent with neurological improvement.
Method: We examined the tracheostomy database of our neurological rehabilitation unit, which reports data from 359 patients with sABI, hospitalized between 2000 and 2012, and all carriers of tracheostomy tube upon admission.
The sample was divided into two groups based on the time gap between the acute event and admission to rehabilitation, an Early Group = 60 days and a Late Group > 60 days. The probability of decannulation, both overall and subdivided by groups, was then evaluated.
Results: The E-Group showed better functional scores at discharge and a better percentage of decannulation compared with the L-Group. The differences were statistically significant, but later decannulations were not unusual: 12% late weanings versus 88% earlier.
The probability of decannulation increases over time and is a factor dependent on the degree of neurological and functional recovery. It is of primary importance to consider these aspects when making comparisons between different centers, defining a product standard or performing benchmarking analyses.
Conclusions: The rehabilitative prognosis for decannulation can be reliably judged only after a suitable and well-defined period of time, because the probability of tube weaning has been shown to be strongly dependent on the amount of time that passes. This study also showed that effective decannulations can be achieved even more than 6 months after the acute event, although with significantly lower probabilities.
Keywords: Severe Acquired Brain Injury; Neurorehabilitation; Tracheostomy, Decannulation; Dysphagia
Abbreviations
sABI: severe Acquired Brain Injury; E-Group: Early Group; L-Group: Late Group; PEG: Percutaneous Endoscopic Gastrostomy; GOS: Glasgow Outcome Scale; FIM: Functional Independence Measure; LOS: Length of Stay; SBDT: Saliva Blue Dye Test; ICU: Intensive Care Unit
Introduction
The probability of removing the tracheostomy tube in patients with severe acquired brain injury (sABI) depends on several factors.
A study conducted by our working group on decannulation prediction, based on a cohort of 463 inpatients with sABI, highlighted how age, saliva aspiration, ABI pathogenesis, altered state of consciousness, and cough score are predictive factors [1].
A more recent study conducted on the same topic involving 327 patients confirmed that age, etiology and consciousness influence the probability of decannulation, adding further factors such as entry scores of consciousness assessment scales (Coma Recovery Scale - Revised, Glasgow Coma Scale) and functional independence scores (Disability Rating Scale, Functional Independence Measure), in addition to the presence of Percutaneous Endoscopic Gastrostomy (PEG) or nasogastric tube [2].
This latter study had a further merit in extending the discussion on decannulation timing and in researching predictors of this variable [2]. The authors stressed that the traumatic etiology is indicative of a short time decannulation (median 50 days) against the remaining etiologies (110 days), a supratentorial trauma location is indicative of decreased decannulation timing, the presence of a nasogastric tube and PEG correlate with decannulation time respectively in a positive and negative direction, and the need and use of mechanical ventilation at admission increase the decannulation time from 95 days to 148 days [2].
An interesting graph from this study shows the decannulation timing of 186 events, and it is surprising to note that in the face of most decannulations occurring between 40 and 140 days (intuitive aspect of the result), there is a minority occurring between 140 and 300 days (peculiar aspect of the result).
The authors were not interested in this aspect and have rightly concentrated their interest on searching for the predictive factors of timing. At the same time, their cohort shows how the probability of decannulation extends over time and expresses the maximum potential over a very extensive period of time (about 1 year from the acute event).
If we focus on the performance of the decannulations, we can see that 186/327 (57%) were achieved at a follow-up time of 300 days (for some patients). What would the performance have been like if the study had been censored 4 months after the acute event? In our opinion, the timing of data censorship is an element that the literature has not yet properly focused on.
In a recent study, three different types of service organization: pre-tracheostomy service (baseline), tracheostomy service alone, and post-tracheostomy care bundle were compared, demonstrating increasing decannulation rates, from 8.2% to 14.5% and up to 26% in the bundled PTC, by implementing multidisciplinary evaluation and decision-making activities [3]. The outcome was collected “before discharge”, with comparable average length of stay between the three organizations at approximately 26 days.
How is it possible to compare two decannulations that take place at such different times?
The time within which “decannulation events” are collected probably represents a crucial element in the rate of success and is also a factor that contributes to generating relevant variability between different studies.
The probability of decannulation lies between the range of 24% of Klein [4] and 77% of Perin [5], going from 31.5% of Mackiewicz- Nartowicz [6] to 46.7% of Perin [7], 54% of Warnecke [8], 57% of Mannini [2], 58% of Citta-Pietrolungo [9], 65% of Matesz [10], 68.5% of De Mestral [11], 70% of Leung [12], 72% of Chan [13], and 73% of Reverberi [1].
In a neurological recovery process, the “time passing” is a very important variable because “as stroke shows various clinical manifestations and recovery processes, swallowing and cough functions also change and improve after a stroke over time” [14].
Some studies have focused on clinical factors that influence the probability of decannulation, including the improvement in swallowing and the strength of the cough reflex [14], the possibility of tracheostomy tube capping, endoscopy assessment of airway patency, instrumental swallowing assessment and the blue dye test [5], mean expiratory pressure, presence of spontaneous cough and, again, cough strength [7], saliva aspiration and cough score [1].
In light of this data and wanting to make a contribution to the scientific discussion on this topic, we reviewed the tracheostomy database of our neurological rehabilitation unit, which reports data between 2000 and 2012, in an attempt to correlate the probability of decannulation with the time elapsed from the acute event.
Methods
All patients admitted to our Neurological Intensive Rehabilitation Unit between December 2000 and March 2012, over 15 years of age, affected by sABI and having a tracheostomy tube in place at admission, were entered in the tracheostomy database.
The tracheostomy database comprises consecutive data collection of tracheostomized inpatients admitted with the goal of weaning from the tube. Patients for whom decannulation was judged not achievable were discharged with the tracheostomy tube in situ; some of them were later readmitted in order to re-evaluate the decannulation option after a longer period of time, using the same decision algorithm and the same criteria defined at the beginning of data collection. For this reason, our database documents both early and late decannulations of patients affected by sABI.
Demographic and clinical data were collected for each patient: the sABI etiology (traumatic, vascular, anoxic, other), the appearance of respiratory infections and obstructive complications, pre- and post-decannulation, removal of the tube and any conditions that impeded it, the severity of the functional impairment assessed using the Glasgow Outcome Scale (GOS) and Functional Independence Measure (FIM) at admission and discharge [15,16].
The temporal elements of clinical evolution identified were the date of acute injury, date of admission into neurological rehabilitation, date of decannulation, date of discharge from rehabilitation. With these elements we could calculate the following time intervals: time before rehabilitation, time of tube in situ (only for weaned inpatients), rehabilitation Length of Stay (LOS), and outcome time (time within which missed weaning was censored or in general discharge from hospital).
In the year 2000, a multidisciplinary panel of experts from our health care facility had defined a shared protocol of decannulation criteria, consisting of pre-requisites and evaluation tools in order to make a rational choice [17,18]:
1. Prerequisites: baseline oximetry not below 90-92% O2; no need for aspirations within 24 hours; “tube capped and cuff deflated” or use of speaking valves must be possible.
2. Clinical assessments: effectiveness of the cough reflex (reflexive or voluntary evocation), oximetry in condition of tube capped, saliva blue dye test (SBDT), swallowing efficacy for liquids and semisolids.
3. Instrument examinations: chest X-ray (optional), laryngeal and tracheal fiberoptic endoscopy to exclude endotracheal complications (mandatory).
The decision to decannulate the patient was based on the following decisional algorithm [17]:
- First choice: Remove the tube only after having recovered autonomy in saliva management and in semisolid consistency food swallowing (at least).
- Second choice: If sufficient swallowing is considered a difficult target to reach in a relatively short time (or an achievable goal only in a long time), but the tube can be removed early from the respiratory point of view, remove the tube as soon as possible, as long as the patient is able to independently manage his salivary secretions in the SBDT test.
In case of respiratory infection or obstructive complications demonstrated with endoscopy, the decision to decannulate was taken only after removing or overcoming the highlighted obstacles (antibiotic therapy, laser therapy, surgery).
All patients underwent swallowing and breathing treatment, neuromotor rehabilitation aimed at enhancing trunk and head control, recovery of the state of consciousness, communication ability and respiratory clearance [17].
To perform the analysis, the sample was split into two groups based on the gap between the acute event and admission to rehabilitation: Early Group ≤ 60 days (E-Group) and Late Group > 60 days (L-Group) (Table 1).
Characteristics
Sample
(349)E-Group
(271)L-Group
(78)p
Male sex, n (%)
220 (63)
176 (65)
44 (56)
°0.16
Age, median (IQR) y
51(12)
50(12)
52(10)
°0.15
Etiology, n (%)
*0.8
Vascular
160(46)
124 (46)
36(46)
Traumatic
116(33)
93(34)
23(29)
Anoxic
23(7)
17(6)
6(8)
Other
50(14)
37(14)
13(17)
FIM admission, median (min max)
20 (18 – 96)
20 (18 – 95)
20(18 – 59)
°0.11
GOS admission, n (%)
*0.19
1. VS
138 (40)
102 (38)
36 (46)
2. Severe D
205 (59)
163 (60)
42 (54)
3. Moderate D
6 (1)
6 (2)
0 (0)
4. Good recovery
0 (0)
0 (0)
0 (0)
Time before rehab median (IQR) d
55 (18)
39 (13)
79 (13)
°0.016
°Wilcoxon rank-sum test (Mann-Whitney)
*Pearson’s chi-squared
FIM: Functional Independence Measure
GOS: Glasgow Outcome Scale
VS: Vegetative state
D: Disability
Time before rehab: gap between acute event and rehabilitation admission; p: statistically significant difference.
Table 1: Patient characteristics.
The statistical analysis was performed using STATA software. Wilcoxon rank-sum test (Mann-Whitney) and Pearson’s chi-squared tests were used respectively for inferences on continuous variables with non-Gaussian distribution, and on dichotomous variables. A logistic regression test was performed to evaluate correlation indices between weaning and the other parameters evaluated. Confidence limits were calculated for each parameter evaluated.
Results
The tracheostomy database includes 359 inpatients, median age 51.45 years, 229 males (64%) and 130 females (36%). Ten individuals died during the stay, none of whom had undergone decannulation. Of the remaining 349 patients, decannulation was performed on 278 (79.6%; 95% CI: 75.4% - 83.9%); in 71 subjects the tube was not removed (20.3%; 95%CI: 16.1% - 24.6%). The E-Group consisted of 271 subjects and the L-Group 78 subjects (see Table 1).
The median “outcome time” was 172 days (min 42 - max 552), a median time of 5.7 months; the median “tube in situ” time was 79 days (min 25 days; 1° IQR 53; 3° IQR 121; max 461), 68 for the E-Group, 151 for the L-Group; the median time before rehabilitation admission was 55 days for the whole sample, 39 days for the E-Group and 79 days for the L-Group.
The E- Group and L-Group showed no statistically significant differences in the admission data, with the exception of the time before rehabilitation (cut-off criterion). As reported in Table 1, they did not show differences in age, sex, etiology, functional impairment or disability on admission.
The difference between the two groups was instead manifested in statistically significant differences in the time of the tube in situ, outcome time, FIM and GOS score at discharge, percentage of decannulation (Table 2). No statistically significant differences were recorded in the remaining outcomes, including respiratory infections and obstructive complications, pre- and post-decannulation, or complications of the tracheal mucosa (Table 2).
Characteristics
Sample
(349)E-Group
(271)L-Group
(78)p
Time of tube in situ, median (min max) d
79 (25 - 461)
68 (25 – 297)
151 (81 – 465)
°0.000
Time outcome, median (min max) d
172 (42 – 552)
154 (40 – 565)
194 (56 – 477)
°0.000
Rehab LOS, median (min max) d
122 (11 – 398)
112 (16 – 489)
136 (11 – 372)
°0.08
FIM discharge, median (min max)
57 (18 – 126)
69.5 (18 - 126)
41 (18 – 126)
°0.000
GOS discharge, n (%)
*0.000
1. VS
53 (15)
32 (12)
21 (27)
2. Severe D
166 (48)
122 (45)
44 (56)
3. Moderate D
71 (20)
64 (24)
7 (9)
4. Good recovery
59 (17)
53 (19)
6 (8)
Decannulation rate, n (%)
278 (79.6)
225 (83)
53 (67.9)
*0.004
Pre-D infectious complications, n (%)
106 (30.4)
76 (28)
30 (38.5)
°0.07
Post-D infectious complications, n (%)
26 (7.4)
18 (6.6)
8 (10.3)
°0.28
Pre-D obstructive complications, n (%)
72 (20.6)
51 (18.8)
21 (26.9)
°0.11
Post-D obstructive complications, n (%)
27 (7.7)
21 (7.7)
6 (7.7)
°0.98
Tracheal granulation tissue, n (%)
79 (22.6)
55 (20.3)
24 (30.8)
°0.051
Tracheal stenosis, n (%)
28 (8)
20 (7.4)
8 (10.3)
°0.41
Tracheomalacia, n (%)
7 (2)
4 (1.5)
3 (3.8)
°0.18
°Wilcoxon rank-sum test (Mann-Whitney)
*Pearson’s chi-squared
Time tube in situ: gap between acute event and decannulation data
Time outcome: gap between acute event and rehabilitation discharge
LOS: Length of stay; p: non statistically significant difference
FIM: Functional Independence Measure
GOS: Glasgow Outcome Scale
VS: Vegetative state
D: Disability
Table 2: Comparison of outcomes.
The probability of decannulation resulted in being significantly dependent upon the nature of the encephalopathy, the severity of the clinical conditions according to the GOS at the moment of discharge, and the pre-decannulation infectious and obstructive complications (see logistic regression in Table 3). No significant associations were recorded between decannulation and duration of rehabilitation, patient gender or age, or clinical picture at admission.
Iteration 0:
log likelihood = -152.38722
Iteration 1:
log likelihood = -89.627537
Iteration 2:
log likelihood = -72.959745
Iteration 3:
log likelihood = -71.186369
Iteration 4:
log likelihood = -71.152472
Iteration 5:
log likelihood = -71.152392
Iteration 6:
log likelihood = -71.152392
Logistic regression
Number of obs = 298
LR chi2
(16) = 162.47
Prob>
chi2 = 0.0000
Log likelihood = -71.152392
Pseudo R2 = 0.5331
Wean |
Coef.
Std.
Err.
z
P>|z|
[95% Conf. Interval]
Sex |
0.169834
0.4912591
0.35
0.730
-0.7930162
1.132684
Inf Compl PRE |
-1.742273
0.4890551
-3.56
0.000
-2.700804
-0.7837429
Inf Compl POST |
-0.2106165
0.7687085
-0.27
0.784
-1.717257
1.296024
Obstr Compl PRE |
-1.77963
0.5912163
-3.01
0.003
-2.938392
-0.6208672
Granulat |
0.7854004
0.709686
1.11
0.268
-0.6055586
2.176359
Stenosis |
0.7425691
0.918258
0.81
0.419
-1.057184
2.542322
Malacia |
1.047252
1.339487
0.78
0.434
-1.578094
3.672597
Obstr Compl POST |
1.086426
0.9895823
1.10
0.272
-0.8531193
3.025972
Pathology |
-0.5564998
0.1968888
-2.83
0.005
-0.9423947
-0.170605
Clinical AD |
-0.3730414
0.5876957
-0.63
0.526
-1.524904
0.778821
Clinical DI |
2.609248
0.577891
4.52
0.000
1.476603
3.741894
FIMad |
0.0728157
0.0416305
1.75
0.080
-0.0087785
0.1544099
FIMdi |
0.0027401
0.0154645
0.18
0.859
-0.0275699
0.03305
Age |
0.0185015
0.0155013
1.19
0.233
-0.0118805
0.0488836
Tpre Rehab |
0.0016138
0.0046232
0.35
0.727
-0.0074475
0.0106752
LOS rehab |
0.0027765
0.0025435
1.09
0.275
-0.0022086
0.0077616
_cons |
-4.328313
1.802404
-2.40
0.016
-7.86096
-0.7956666
Table 3: Logistic regression of dichotomous “decannulation” variable.
In detail, post-anoxic (39.1%) and vegetative state patients at admission (63.7%) showed the least absolute probability of being weaned from a tracheostomy tube, and the presence of respiratory infections (60.3%) and tracheal obstructive complications (59.7%) that preceded the decannulation procedure also led to inferior results.
On the contrary, the probability of decannulation was much higher in the case of traumatic etiology (85.3%), severely disabled patients at admission according to the GOS (89.7%), absence of predecannulation respiratory infections (88%) and of pre-decannulation tracheal obstructive complications (84.8%).
The frequency of weaning from the tracheal tube showed a positive asymmetric distribution (Figure 1). Most of the events took place within 6 months of the acute event, though late decannulation was not unusual: 246 of 278 early weanings (88%) versus 32 late (12%).
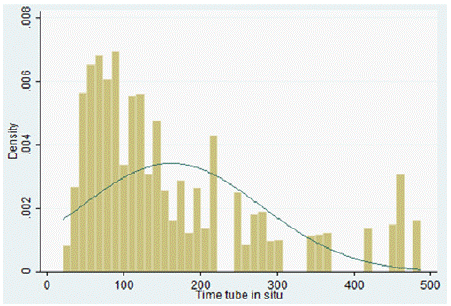
Figure 1: Time of tube in situ distribution (in days).
Discussion
The decannulation timing data collected in our tracheostomy database are quite similar to those of Mannini et al. [2] and our timing chart is quite similar to the one published by them. Both graphs show a Gaussian-like distribution of early decannulations, followed by an asymmetrical tail that lasts over time (in our database there is one case of decannulation that occurred more than one year after the acute event).
These data confirm the point of view expressed by several authors that if decannulation probability is influenced by an improvement in factors that occurs over time, such as swallowing and cough [14,5,7], then the time passing plays an important role in the result.
Enrichi reports 77% success in neurological patients in a “time from cannula placement to assessment” of 66.1 days for decannulated patients, and of 82.5 days for non-decannulated patients [5]. Perin reports 46.7% success without indicating a precise period of time within which the outcome was recorded [7]; Mah reports 26% success evaluated on average 26 days after the acute event [3].
Our database reports data similar to those of Enrichi, slightly worse in terms of median “tube in situ” (79 days vs 66.1) but slightly better in terms of decannulation percentage (79.6% vs 77%) [5].
It is more difficult for us to make a comparison with Mah’s data [3], because in our database only 3 patients were decannulated within the 26th day after the acute event. Our patients are a selected population among patients not considered decannulable in the acute phase, or for whom early weaning was not successful.
At the same time, they do not represent a continuous cohort of patients entering our ward from the ICU, and for this reason the probability of decannulation was higher than that published by Enrichi [5].
The comparison between different studies is difficult because the data, in order to be compared, must guarantee the same timing conditions, but also the same conditions of admitting severity, etiology and neurological recovery potential. A more standardized comparison would require further information on the etiology, the degree of initial functional impairment (GOS and FIM), the presence of infectious and/or obstructive complications before decannulation (see Table 3), but also the severity of dysphagia and the cough strength [1]. We have in fact recorded the variability of the probability of weaning from the tracheal tube in different levels of severity of clinical conditions, as described in the results and as reported in various articles recently published [1,7,5].
The data we have collected show that the probability of decannulation decreases over time and presents a positive asymmetric distribution. In particular, if it is true that most of the decannulation events took place within 6 months from the acute event, it is equally true that at least 32 decannulations (12%) occurred in over 6 months, nearing one year and in some cases near the second year.
Late decannulations should not be considered timely and temporally related to clinical improvement in a stringent manner. In most cases, in fact, they involved patients who, having were readmitted to the hospital, were evaluated as improved in several skills (swallowing, cough strength, state of consciousness or head and trunk control from sitting) in order to reconsider the goal of decannulation previously considered “unattainable” based on the same rules defined in the methods. For this reason, late decannulations in our tracheostomy database should not be judged in terms of timeliness, but rather in terms of opportunities later available to patients who were not considered eligible for decannulation at earlier times.
The removal of the tracheostomy tube represents an important goal in terms of both quality of life and reducing complications, as well as the cost of care. For this reason, we maintain our willingness to carry out short, even late, admissions aimed at achieving targets that are not reachable early, such as weaning from the tracheostomy tube. Therefore, our tracheostomy database represents a collection of episodes of hospitalization with a precise goal (decannulation) rather than a set of data regarding patients in the acute rehabilitation phase, and from this point of view there are some patients recorded twice in the database: in the initial phase and then in the late phase.
When the database was collected, a temporal analysis such as the one presented in this study was not foreseen, and the Early and Late groups were not separated based on a precise indicator defined in advance. In this study, to be able to carry out the analysis by groups, we used a mathematical artifice (cutoff point: 60 days of gap between acute event and date of rehabilitation admission), which presents some limits and requires some discussion.
First of all, it must be noted that both the E-group and the L-group include patients with late decannulations, even if they represent outsiders. Substantially, the groups are represented by patients who maintained the cannula in situ for decidedly different times (median of 68 vs 151 days).
Furthermore, in the Tracheostomy database, the health path preceding admission into rehabilitation was not specified (for example, whether it was a first rehabilitation admission or a second late admission), so it is not possible to analyze it. For this reason, the L-group could include patients at the first admission into acute rehabilitation even if later than average, patients with more complicated health paths and several steps in acute wards, patients admitted into rehabilitation extra time for decannulation. As a result, the L-group is realistically a heterogeneous group, while the E-Group with greater certainty represents a homogeneous group of patients at the first admission in acute rehabilitation after the acute event.
Finally, the choice of the 60-day cutoff is definitely optional, but this arose from the need to distinguish a true group of early admissions from a group of late and spurious admissions, with the knowledge that most of the patients who access our rehabilitation unit are actually at their first rehabilitative access after an acute event.
In all cases, it is interesting to note that the two groups, selected in the aforementioned way, were totally comparable from the point of view of the input variables (see Table 1), and showed statistical differences only in terms of the severity of the functional impairment at discharge and the probability of decannulation (as well as differences in terms of timing, which are a direct consequence of the cut-off criterion) (see Table 2).
In other words, a temporal cut-off that separates two groups based on the timeliness of access to rehabilitation (one early and one late) produces a direct influence on both the probability of functional recovery and the probability of decannulation in favor of the E-Group.
However, the biggest surprise is counterintuitive, and it can be seen in the L-Group: the conditions for a decannulation can also be recorded belatedly, even in a population with less probability of a functional recovery (see Figure 1).
This means that the recovery of neurological functions, even if late, even concerning a population of more serious inpatients with fewer prospects for neurological recovery, influences the probability of decannulation as a variable distributed over time.
It also means that a rehabilitative prognosis for decannulation can be judged reliable only after a suitable and well-defined period of time.
Conclusions
This study confirms a well-known fact in the rehabilitation field, namely that neurological recovery takes place over time and different rehabilitation goals require time to be realized.
The probability of decannulation has been shown to be strongly dependent on the time passing between the acute event (close to the positioning of the cannula) and the weaning time, the reason being that in order to decannulate a patient it is necessary to increase the effectiveness of swallowing and the strength of the cough. There are various rehabilitative strategies to achieve this result, but above all neurological improvements occur when the neuronal plasticity is active and strong, and this brain property requires biological time to be realized: a time passing.
This study also showed that successful decannulations can be achieved even more than 6 months after the acute event, although with significantly lower probabilities, in specific patients who have improved over time.
Studies on the probability of decannulation must therefore clearly explain the timing within which the outcome is detected, especially if they want to compare the results of different centres, define a product standard or perform a benchmarking analysis.
References
- Reverberi C, Lombardi F, Lusuardi M, Pratesi A, Di Bari M. Development of the Decannulation Prediction Tool in Patients With Dysphagia After Acquired Brain Injury. J Am Med Dir Assoc. 2019; 20: 470-475.e1.
- Mannini A, Hakiki B, Liuzzi P, Campagnini S, Romoli A, Draghi F, et al. Datadriven prediction of decannulation probability and timing in patients with severe acquired brain injury. Comput Methods Programs Biomed. 2021; 209: 106345.
- Mah JW, Staff II, Fisher SR, Butler KL. Improving Decannulation and Swallowing Function: A Comprehensive, Multidisciplinary Approach to Post- Tracheostomy Care. Respir Care. 2017; 62: 137-143.
- Klein AM, Howell K, Straube A, Pfefferkorn T, Bender A. Rehabilitation outcome of patients with severe and prolonged disorders of consciousness after aneurysmal subarachnoid hemorrhage (aSAH). Clin Neurol Neurosurg. 2013; 115: 2136-2141.
- Enrichi C, Battel I, Zanetti C, Koch I, Ventura L, et al. Clinical Criteria for Tracheostomy Decannulation in Subjects with Acquired Brain Injury. Respir Care. 2017; 62: 1255-1263.
- Mackiewicz-Nartowicz H, Mackiewicz-Milewska M, Lach S, Szymanska- Skrzypek A, Owczarek A, et al. Decannulation factors in patients after serious brain injuries. Adv Pall Med. 2008; 7: 69-72.
- Perin C, Meroni R, Rega V, Braghetto G, Cerri CG. Parameters Influencing Tracheostomy Decannulation in Patients Undergoing Rehabilitation after severe Acquired Brain Injury (sABI). Int Arch Otorhinolaryngol. 2017; 21: 382-389.
- Warnecke T, Suntrup S, Teismann IK, Hamacher C, Oelenberg S, Dziewas R. Standardized endoscopic swallowing evaluation for tracheostomy decannulation in critically ill neurologic patients. Crit Care Med. 2013; 41: 1728-1732.
- Citta-Pietrolungo TJ, Alexander MA, Cook SP, Padman R. Complications of tracheostomy and decannulation in pediatric and young patients with traumatic brain injury. Arch Phys Med Rehabil. 1993; 74: 905-909.
- Matesz I, Dénes Z, Belinszkaja G, Frey E, Nagy H, et al. Bronchoscopyguided decannulation of tracheostomy in patients with brain injury. Orv Hetil. 2014; 155: 1108-1112.
- de Mestral C, Iqbal S, Fong N, LeBlanc J, Fata P, et al. Impact of a specialized multidisciplinary tracheostomy team on tracheostomy care in critically ill patients. Can J Surg. 2011; 54: 167-172.
- Leung R, MacGregor L, Campbell D, Berkowitz RG. Decannulation and survival following tracheostomy in an intensive care unit. Ann Otol Rhinol Laryngol. 2003; 112: 853-858.
- Chan LY, Jones AY, Chung RC, Hung KN. Peak flow rate during induced cough: a predictor of successful decannulation of a tracheotomy tube in neurosurgical patients. Am J Crit Care. 2010;19: 278-284.
- Park MK, Lee SJ. Changes in Swallowing and Cough Functions Among Stroke Patients Before and After Tracheostomy Decannulation. Dysphagia. 2018; 33: 857-865.
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1: 480-484.
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987; 1: 6-18.
- Reverberi C, Lombardi F. Tracheostomy and dysphagia in severe brain injury. Choose, evaluate and rehabilitate. Reverberi C, Lombardi F. Editors. Editions of Cerro September 2007.
- Bargellesi S, Reverberi C, De Tanti A, Pregno S. The management of the tracheostomy tube in people with severe acquired brain injury: consent to a shared protocol. MR Italian Journal of Rehabilitation Medicine. 2013; 27: 9-16.