
Research Article
Austin J Plant Biol. 2015;1(1): 1003.
Isolation and Evaluation of Endophytic Bacteria Against Fusarium Oxysporum f. sp. Cucumerinum Infecting Cucumber Plants
Ozaktan H¹*, Çakir B², Gül A¹, Yolageldi L², Akköprü A³, Fakhraei D¹ and Akbaba M¹
¹Department of Plant Protection, University of Ege Faculty of Agriculture, Turkey
²Department of Horticulture, University of Ege, Faculty of Agriculture, Turkey
³Department of Plant Protection University of Yüzüncü Yil, Turkey
*Corresponding author: Birsen Çakir, Department of Horticulture, Faculty of Agriculture, Ege University, Bornova, Izmir, Turkey
Received: August 04, 2014; Accepted: February 02, 2014; Published: February 23, 2015
Abstract
The aim of this study was to isolate, within cucumber plants, Endophytic Bacterial (EB) isolates that can provide significant biological control of Fusarial wilt of cucumber caused by Fusarium Oxysporum f. sp. Cucumerinum (FOC) and enhance plant growth under in vitro conditions. The endophytic bacteria were isolated from the internal tissues of roots, leaves and stems of healthy cucumber plants. In this study, we isolated 112 EB strains from internal tissues of healthy cucumber plants grown in greenhouse and field conditions in Turkey. We determined several phenotypic properties of EB strains and found approximately equal numbers of Gram-negative and Gram-positive strains. These isolates were screened in vitro for their plant growth promoting traits such as production of Indol 3-Acetic Acid (IAA), Hydrogen Cyanide (HCN), siderophore, phosphate solubilization and antagonistic activity against FOC. More than 30% of the EB strains produced detectable levels (20-125 μg ml-1) of IAA in culture filtrates. 46 % of EB strains exhibited siderophore production ranging from 3 to 19 mm zones. HCN production was more common trait of Pseudomonas strains (16%). Solubilization of phosphate was detected by 29% in the EB isolates. More than 53% of the EB strains inhibited the mycelial growth of FOC on PDA plates. The strains CC29/3 and CC25/2 were more active compared to other against FOC. Majority of isolated endophytes were not only able to suppress pathogenic fungi, but could also improve seed germination and plant growth. Finally, these strains may be candidates for biological control and plant growth promotion.
Keywords: Fusarium oxysporum f. sp. cucumerinum; Endophytic bacteria; Biological control
Abreviations
FOC: Fusarium Oxysporum f. sp. Cucumerinum; EB: Endophytic Bacteria; IAA: Indol 3-Acetic Acid, HCN: Hydrogen Cyanide
Introduction
Fusarium Oxysporum f. sp. Cucumerinum (FOC), which is a soilborne fungus, causes wilting and death of cucumber plants grown in greenhouse [1]. The symptoms include necrotic lesions of the stem base, foliar wilting and eventually plant death [1,2]. FOC has been identified in all cucumber growing regions around the world, including Turkey [3,4] and has been documented as an important economic threat to cucumber producers [3,5-7]. It is difficult to control of FOC since the pathogen could cause systemic invasion and move in the cucumber plant tissues by xylem vessels [1]. Chemical control methods which are effective against Fusarium root and stem rot of cucurbits are limited [8]. Therefore, control strategies focus mostly on preventing the pathogen from being introduced into disease-free areas, and the development of disease resistant varieties [9]. Hovewer, the development of new pathogenic races of FOC limits use of disease resistant varieties [3]. To date, there is no resistant cucumber cultivars to FOC. In response to environmental and health concerns about extended use of pesticides, there is considerable interest in finding alternative control approaches for use in integrated pest management strategies for crop diseases.
Biological control offers potential alternatives to combat many soil-borne pathogen, including Fusarium oxysporum [10]. The biological control of Fusarium wilt of cucumber caused by FOC was obtained using siderophore fluorescent pseudomonads in Turkey [11].
The use of Endophytic Bacteria (EB) strainsto control plantpathogenic bacteria and fungi is receiving increasing attention as a sustainable alternative to synthetic pesticides. EB strains, which live inter- and intracellularly in plants without inducing pathogenic symptoms, interact with the host biochemically and genetically. EB may play many important beneficial roles in the metabolism and physiology of the host plant, including fixing atmospheric nitrogen, sequestering iron from the soil, solubilizing phosphates, synthesizing plant – growth hormones, and suppressing of ethylene production by 1-Amino Cyclopropane-1-Carboxylate (ACC) deaminase, degrading toxic compounds, inhibiting strong fungal activity and antagonizing bacterial pathogens [12-14]. The internal plant tissues provide a protective environment for endophytic bacteria, which colonize an ecological niche similar to plant pathogens. Endophytes, like Pseudomonas, Agrobacterium, Bacillus, Burkholderia and Enterobacteria, have been isolated from root nodules in various leguminous plants including alfalfa, clover, soybean pigeon pea, etc [15] since 1902 [16-18]. Available reports indicated improved plant yield and health under greenhouse conditions (measured as an increase in root wet weight and nodulation) when co-inoculated with nodule endophytes compared to inoculation with rhizobia alone.
In order to reduce the input of pesticides and fertilizers and to make an eco-friendly agriculture, it will be important to develop inocula of biofertilizers, and biopesticides. The main aims of this study are: (i) to collect different EB from different area in Turkey, (ii) to screeen these bacteria for a number of plant-beneficial traits, (iii) to test the selected potentially beneficial strains for their abilities to promote growth of cucumber and to control FOC caused by Fusarium oxysporum f. sp. Cucumerinum.
Materials and Methods
Isolation of endophytic bacteria
The endophytic bacteria were isolated from the internal tissues of roots, leaves and stems of healthy cucumber plants, which were surface-sterilized by sequential immersion in 70% ethanol for 5 min and a solution of 5% sodium hypochloride for 10 min. Then the samples were rinsed three times in sterile distilled water to remove surface sterilizing agents prior to obtain bacterial isolates. Bacterial strains were isolated by two different techniques such as trituration of leaves and imprinting of stem and root tissues onto Triptic Soy Agar (TSA). Surface sterility test was performed for each of the samples to ensure the elimination of surface microorganisms. If no bacterial growth occurred in the sterility test, the recovered bacteria were considered to be endophytes. Single colonies were isolated and maintained in pure cultures at -800C in 15% (v/v) glycerol [19].
Phenotypic characterization of bacterial strains
Colonies of bacterial isolates were characterized for the following traits: color, form, elevation, margin, diameter, surface, opacity, and texture. The Gram reaction was performed by using a 3% KOH test [20]. Endophytic Bacteria (EB) were tested for Hypersensitive Response (HR) on tobacco leaves. EB strains, showed HR (+) on tobacco leaves were considered as possible plant pathogens and were not taken for further tests [21.22].
In vitro screening for antagonistic activity
All EB isolates were screened for their in vitrobiocontrol activity toward FOC on Potato Dextrose Agar (PDA) using a dual-culture technique [23]. The plates inoculated with the pathogen alone were maintained as control. The mycelial disc (5 mm) from 7 days old culture of FOC was placed on one side of the plate containing PDA medium, and then EB strains were streaked on the opposite side of the plate by the help of sterilized inoculation needle. Three replications were performed for each treatment. The plates were incubated at room temperature for seven days. The inhibitory effects of EBstrains on the linear growth of FOC were determined. The percent of inhibited FOCwas calculated by comparison with fungal growth in control plates.
In vitro characterization of EB strains for plant growth promotion
The EB strains were screened by in vitro assays for the production of the following functional traits: hydrogen cyanide, HCN [24]; phosphate solubilization [25] and Indole 3- Acetic Acid, IAA [26]; plant growth promotion and siderophore production [27]. All experiments were replicated twice for each of the strains.
Effect on seed germination and seed vigor index
EB strains, which were found successful by in vitro assays for plant growth promotion and antagonistic activity to FOC, were further analyzed for seed emergence (cv. Gordion) and measured for Vigor Index (VI). For bacterization, seeds were surface sterilized with 1% sodium hypochlorite for 1 min and soaked in EB suspensions ammended with 1% Carboxy Methyl Cellulose (CMC). After bacterization, the seeds were placed onto sterile filter paper moistened with Sterile Distilled Water (SDW) in petri plates (three plates with 10 seeds/plate) and incubated at room temperature. Control plates were arranged in a similar way, except that they were treated only with 1% CMC. For each isolate, effects on seed germination were measured by counting the number of fully germinated seeds per plate and comparing that with that of the control plates. After 5 days, the vigor index for each treatment was calculated by using the formula: VI= Percent germination X (seedling length + root length)as described previously [28].
Results and Discussion
Isolation of EB strains
We observed that the inner tissues of healthy cucumber plants were very rich for EB colonization Trituration and imprinting of the plant tissues were the reliable and easy isolation techniques for EB. A total of 44 healthy cucumber plants including 34 plants grown in greenhouse and 10plants grown in field were obtained from different sampling areas in Turkey. Surface sterility tests were performed for each sample to monitor the efficiency of the disinfestation procedure during isolation. If no bacterial growth occurred in the sterility test, the recovered bacteria were considered to be endophytes. Here, 112different endophytic colonizing bacterial strains were isolated from healthycucumber plants grown in greenhouse and field conditions. On the basis ofphenotypic identification tests such as some cultural, morphological and biochemical characteristics, a total of 104 endophytic bacterial strains were groupedinto Gram (-) bacteria, and Gram (+) bacteria. Most of the EB strains were flurescent pseudomonads, Gram negative (66%) and rest Gram positive (Table 1). Among Gram-negative soil bacteria, Pseudomonas is the most abundant genus in the rhizosphere [29]. Root-associated Pseudomonas spp. strains have long been known to be beneficial to plants attribute to their Plant-Growth Promotion Effect (PGPE) or their potential as biological control agents. In addition, endophytic Pseudomonas spp. can also indirectly induce PGPE by controlling phytopathogens or pathogenic fungi using mechanisms such asproducing antibiotic factors [30,31], enhancing competition for colonization sites [32], and induction of systemic resistance [33]. The diversity of EB reported here has many similarities with the EB isolated from other plants. Agrobacterium, Arthobacter, Bacillus, Chryseobacterium, Enterobacter, Pseudomonas and Sterotrophomonas were commonly identified from roots of cucumber, sugar beet, corn, and lemon [34].
EB isolates
Bacterial Species
The percent inhibition of mycelial development of FOC1, 2
IAA3
(µg/ml)
Gram
Staining
Floresencent pigmentation
HCN4
Siderophore
production
Phosphate
solubilization
CB1/1
2
38,1
31
-
-
-
1 mm
0
CB1/2
3
15,7
25
-
-
-
0 mm
0
CB2/1
4
44,7
30
+
-
-
0 mm
0
CB2/2
5
50
100
-
-
-
12 mm
0
CB2/3
6
57,8
44
-
-
-
10.5 mm
0
CB3
7
30,2
17
+
-
-
0 mm
0
CB4
8
28,9
37
-
-
-
5 mm
3 mm
CC4
9
26,6
27
-
-
-
1 mm
1 mm
CB5/2
11
40,7
26
-
-
-
7 mm
1 mm
CB7
12
34,2
22
-
-
-
5 mm
2 mm
CC7/1
13
56,7
12
-
+
-
13 mm
0 mm
CC7/2
14
53,9
30
-
-
-
5 mm
2,5 mm
CB8/1
15
27,6
37
-
-
-
5 mm
3 mm
CB8/2
16
18,5
32
+
-
-
10 mm
0
CB9/2
18
31,5
13
-
-
-
7 mm
1 mm
CB9/3
19
27,6
33
-
-
-
12 mm
0
CA10
20
39,4
6
+
+
-
0 mm
0 mm
CB10
21
32,8
6
-
-
-
12 mm
9 mm
CC13
22
0
6
+
-
-
0 mm
0
CA13/1
23
15,7
6
+
-
-
0 mm
0
CA13/2
24
0
5
-
-
-
0 mm
0
CB13
25
18,5
15
+
-
-
0 mm
0
CA15
26
0
3
-
-
0 mm
2 mm
CB15/1
27
0
5
+
-
-
0 mm
0
CB15/2
28
23,6
10
-
-
-
0 mm
0
CA17/1
29
14,4
12
-
-
-
0 mm
0
CA17/2
30
28,9
6
-
-
-
9 mm
0
CA17/3
31
26,3
9
-
+
+
7 mm
0
CA17/4
32
44,7
27
+
-
-
2 mm
0
CB17/1
33
0
6
+
-
-
0 mm
0
CB17/2
34
0
0
-
-
-
0
0
CA18
35
17,1
7
+
-
-
6.5 mm
0
CB18/1
36
0
6
+
-
-
0 mm
1 mm
CB18/2
37
14,4
7
-
-
-
7 mm
1 mm
CB20/1
38
0
7
+
-
-
0 mm
0
CB20/2
39
0
11
-
+
-
8 mm
3 mm
CB20/3
40
35,7
9
-
-
1 mm
1 mm
CC23/1
42
4,2
7
-
-
-
2 mm
2 mm
Table 1: Overview of plant-beneficial traits of selected endophytic bacteria.
CC23/2
43
0
10
+
-
-
1 mm
2 mm
CA24
44
0
6
+
-
-
0mm
1 mm
CB24
45
30
18
+
-
-
1 mm
2 mm
CA25
46
14,2
32
-
-
-
3 mm
0
CB25
47
0
12
+
-
-
3 mm
1 mm
CC25/1
48
7
26
-
+
-
14 mm
0
CC25/2
49
64,2
34
-
+
-
9 mm
1 mm
CC26
50
58,5
16
-
-
-
0 mm
0
CA27/1
51
0
9
-
-
-
0 mm
0
CA27/2
52
8,5
9
-
+
+
11 mm
4 mm
CC27
53
18,5
9
-
+
+
13 mm
4 mm
CB27/1
54
0
7
+
-
-
0 mm
0
CB27/2
55
0
6
+
-
-
0 mm
0
CA28/1
56
45,7
13
-
-
-
12 mm
0
CA28/2
57
34,2
35
-
+
-
6 mm
0
CA28/3
58
15,7
15
-
+
-
19 mm
0
CB28
59
24,2
0
+
-
-
0 mm
0
CA29/1
60
27,1
25
-
+
-
3 mm
2 mm
CA29/2
61
27,1
14
-
+
-
16 mm
0
CC29/2
65
7
7
-
-
-
0 mm
0
CC29/3
66
62,8
12
+
-
-
0 mm
0
CC30
68
17,1
24
-
+
-
16 mm
2 mm
CB31
69
0
5
+
-
-
-
0
CA32/1
70
0
35
-
-
-
2 mm
0
CA32/2
71
50
35
+
-
-
8 mm
0
CA33/1
72
48
7
+
-
-
4mm
0
CA33/2
73
44
14
-
+
-
6 mm
3 mm
CA34
74
20
5
+
-
-
1 mm
0
CB34
75
0
5
+
-
-
1mm
0
CC35/1
76
14
16
-
+
-
8 mm
0
CC35/2
77
43
7
+
-
-
2 mm
0
CC35/3
78
0
8
-
-
-
12 mm
0
CA36
79
14
8
+
-
-
1 mm
0
CB36/1
80
43
125
-
-
-
7 mm
4 mm
CB36/2
81
25
20
+
-
-
10 mm
0
CC37/1
82
22,2
5
+
-
-
3 mm
0
CC37/2
83
15
45
-
-
-
7 mm
6 mm
CC37/3
84
42
15
+
-
-
5 mm
0
CA38
85
63
5
+
-
-
6 mm
0
CB38/1
86
0
6
+
-
-
4 mm
0
CB38/2
87
31
50
-
-
-
6 mm
1 mm
CA39/1
88
0
12
-
-
-
12 mm
0
CA39/2
89
32,6
13
+
-
-
4 mm
0
CB39
90
33,3
12
+
-
-
1 mm
0
Table 1 of 2:
CC39 /1
91
11,1
8
+
-
-
3 mm
0
CC39 /2
92
0
15
+
-
-
1 mm
0
CC39 /3
93
42
39
+
-
-
1 mm
0
C40
94
31,5
12
+
-
-
3 mm
0 mm
CB40 /1
95
16,4
11
-
+
-
11 mm
3 mm
CB40 /2
96
24,6
18
-
+
-
19 mm
0
CC40 /1
97
10,9
5
-
-
2 mm
0
CC40 /2
98
23,2
32
-
-
-
4 mm
1,5 mm
CA41 /1
99
17,8
6
+
-
-
1 mm
0
CA41 /2
100
9,5
11
-
-
-
2 mm
0
CA41 /3
101
17,8
9
+
-
-
2 mm
0
CC41 /1
102
26
8
+
-
-
0 mm
0
CC41 /2
103
19,1
41
-
-
-
3 mm
3,5 mm
CA42
104
10,9
8
-
-
-
0 mm
0
CB42 /1
105
17,8
26
+
-
-
1 mm
0
CB42 /2
106
17,8
6
+
-
-
0 mm
0
CC42 /1
107
20,5
41
-
-
-
1 mm
5 mm
CC42 /2
108
30,5
15
-
-
-
1 mm
1 mm
CC43
109
24,6
7
+
-
-
0 mm
1 mm
CA44 /1
110
34,2
6
+
-
-
0 mm
0
CA44 /2
111
26
8
-
-
-
1 mm
0
CC44
112
31,5
8
-
+
-
6 mm
1.5 mm
1The values show the percent inhibition of mycelial development of FOC compared to non treated positive control plates of FOC
2 The valuesare the mean of four replicate plates
3Auxin level after growth in medium supplemented with/without trytophan
4Hydrogen cyanide production
5Mean vigor index value of non treated negative control was 4947
Table 1 of 3:
In vitro plant growth promoting traits
One of the mechanisms of stimulation of plant growth by bacteria involves the production of phytohormones such as auxins, giberellins and cytokinins. Auxins are known to be essential for plant physiology directly affecting the root and shoot architecture. More than 30 % of the EB strains produced detectable levels (20- 125 μg ml-1) of IAA in culture filtrates (Table 1). IAA production was highest in the Pseudomonas followed by Bacillus isolates. Plant growth promotion mediated by endophytic bacteria may be exerted by several mechanisms, e.g. synthesis of siderophores, solubilisation of minerals such as phosphorous [12,13,35]. Siderophore production was exhibited by 46 % strains ranging from 3 to 19 mm zones on CAS agar (Table 1). Solubilization of phosphate was detected in29 % ofthe EB strains ranging from 1 to 9 mm zones (Table 1). Most EB strains were HCN negative on TSA, with or without glycine. Only three Pseudomonas isolates showed detectable cyanide production by changing color from yellow to brown around its colonies.In this study, we showed that EB strains were very promising in respect to in vitro plant growth promotion parameters.
Effect on fungal growth
A total of 112 strains of EB were tested for their in vitro antagonistic activity against FOC on PDA plates. 53% of the EB strains showed antagonism against FOC on PDA plates producing inhibition zones by dual plate test (Table 1). The inhibitory rates varied from 20% to 64% depending on EB strains (Table 1).Most of the EB isolates, which were HCN negative on TSA showed antibiosis against FOC in vitro. Endophytic bacteria isolated from potato roots expressed high levels of hydrolytic enzymes such as cellulase, chitinase and glucanase [36]. Our results showed that the EB strains were effective against FOC and produced inhibitory metabolites other than hydrogen cyanide.
Effect on seed germination
Thirty eightisolates out of 112 EB, considered as successful for in vitro plant growth promoting traits and bicontrol activities toward FOC were analyzed for their effects on seed emergence on cucumber seeds and VI. Some of the EB strains had no apparent effect on seed germination and VI, where as others, when applied individually, caused suppression of seed germination in vitro and seedling growth compared to that of the control plates (Table 1). For example, among 38 EB strains, which were used in plate assay, strains CC37/2 and then CB38/2 were the best ones on enhancement of VI, while strains CA29/1, CA28/2, CB36/2 and Ca38 did not have any effect on seed germination or VI (Figure 1). Consistent with our results, some bacterial endophytic isolates from healthy plants inhibited the growth of tomato seedlings in reinoculation assays, possibly through the production of certain metabolites [37]. In plate assay, CA33/2 and CB8/1strains had the lowest values of VI (Figure 1). We observed that 40% of tested EB strains improved the VI of cucumber seedlings compared to that treated with CMC (1% w/v) only (Figure 1). Approximately 54 % of tested EB isolates had a strong potential for promoting seed germination and VI comparing to control plates. [19] described that the isolates of endophytic bacteria significantly improved seed germination and plant growth of oilseed rape and tomato.
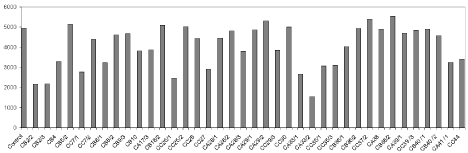
Figure 1: Vigority Index (VI) of germinated cucumber seeds coated with EB strains.
Conclusion
In conclusion, EB can be isolated by surface-sterilized method. A total of 112 EB strains were recovered from cucumber plants.Present study showed high activity of EB strains against FOC. The strains CC29/3 and CC25/2 were more active compared to other strains against FOC. Furthermore, majority of isolated endophytes were not only able to suppress pathogenic fungi, but could also improve seed germination and plant growth These strains may be candidatesfor biological control and plant growth promotion.
Acknowledgement
This study was supported by national grants from the Scientific and Technological Research Council of Turkey (TUBITAK-COST 111 O 505) and Ege University Science-Technology Research and Application Center (EBILTEM). The authors thank the contributions of the participants in the EU-COST Action FA1103.
References
- Owen J H. Fusarium wilt of cucumber. Phytopathology. 1955; 45: 435–439.
- Owen JH. Cucumber wilt, caused by Fusarium Oxysporum f. sp. Cucumerinum N.F. Phytopathology. 1956; 46: 153–157.
- Martyn RD. Fusarium wilt of cucumber. TA Zitter DL, Hopkins, CE Thomas, editors. In: Compendium of cucurbit diseases. St. Paul: The America Phytopathological Society Press. 1996.
- Wicks TJ, Volle D and Baker BT. The effect of soil fumigation and fowl manure on populations of Fusarium oxysporum f. sp. cucumerinum in greenhouse soil and on the incidence of cucumber wilt. Agricultural Record. 1978; 5: 4-8.
- Jenkins SF, Wehner TC. Occurrence of Fusarium oxysporum f. sp. cucumerinum on greenhouse-grown Cucumis sativus seed stocks in North Carolina. Plant Disease. 1983; 67: 1024–1025.
- Martínez R, Aguilar MI, Guirado ML, Álvarez A, Gómez J. First report of fusarium wilt of cucumber caused by Fusarium oxysporum in Spain. Plant Pathology. 2003; 52: 410.
- Neshev G. Major soil-borne phytopathogens on tomato and cucumber in Bulgaria, and methods for their management. Rome: Food and Agriculture Organization of the United Nations (FAO). 2008: 1-22.
- Song W, Zhou L, Yang C, Cao X, Zhang L, LiuZ. Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop protection. 2004; 23: 243-247.
- Lemanceau P, Alabouvette C. Suppression of Fusarium wilts by fluorescent pseudomonads: mechanisms and applications. Biocontrol Science and Technology. 1993; 3: 219-34.
- Spadaro D and Gullino ML. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop Protection. 2005; 24: 601-613.
- Altin N, Bora T. Research on the biological control of Fusarium wilt of cucumber (Fusarium oxysporum f.sp. cucumerinum) in greenhouse PhD. Thesis in Plant protection. 2004; 111.
- Hurek T, Reinhold-Hurek B. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J Biotechnol. 2003; 106: 169-178.
- Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001; 52: 487-511.
- Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008; 278: 1-9.
- Rajendran G, Sing F, Desai AJ, Archana G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. See comment in PubMed Commons below Bioresour Technol. 2008; 99: 4544-4550.
- Zakhia F, Jeder H, Willems A, Gillis M, Dreyfus B, de Lajudie P. Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microb Ecol. 2006; 51: 375-393.
- Kan FL, Chen ZY, Wang ET, Tian CF, Sui XH, Chen WX. Characterization of symbiotic and endophytic bacteria isolated from root nodules of herbaceous legumes grown in Qinghai-Tibet Plateau and in other zones of China. Arch Microbiol. 2007; 188: 103-115.
- Li JH, Wang ET, Chen WF, Chen WX. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem. 2008; 40: 238-246.
- Nejad P, Johnson PA. Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol Control. 2000; 18: 208–215.
- Suslow TV, Schroth MN, Isaka M. Application of a rapid method for Gram differentiation of plant pathogenic and saprophytic bacteria without staining. Phytopathology. 1982; 72: 917–918.
- Lelliot RA, Stead DE . Methods for Diagnosis of Bacterial Diseases of Plants. Blackwell. UK. 1987.
- Schaad NW, Jones JB, Chun W. Laboratory guide for identification of plant pathogenic bacteria. Third Edition, APS: New York. 2001.
- Trivedi P, Pandey A, Palni LM. In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol Res. 2008; 163: 329-336.
- Bakker AW, Schippers B. Microbiyal cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth – stimulation. Soil Biol. Biochem. 1987; 19: 451-457.
- Pikovskaya RE . Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologia. 1948; 17: 362-370.
- Bric JM, Bostock RM, Silverstone SE. Rapid in situ assay for indoleacetic Acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol. 1991; 57: 535-538.
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987; 160: 47-56.
- Abdul- Baki A, Anderson JD. Vigor determination in Soybean seed by multiple criteria. Crop Sci. 1973; 13: 630-633.
- Bardas GA, Lagopodi A, Kadoglidou K, TzavellaKlonari K. Biological control of three Colletotrichum lindemuthianum races using Pseudomonas chlororaphis PCL1391 and Pseudomonas fluorescens WCS365. Biol Control. 2009; 49: 139–145.
- Jousset A, Rochat L, Scheu S, Bonkowski M, Keel C. Predator-prey chemical warfare determines the expression of biocontrol genes by rhizosphere-associated Pseudomonas fluorescens. Appl Environ Microbiol. 2010; 76: 5263-5268.
- ValletGely I, Novikov A, Augusto L, Liehl P, Bolbach G, Péchy-Tarr M, et al. Association of hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, with cyclic lipopeptide production. Appl Environ Microbiol. 2010; 76: 910–921
- Wensing AD, Braun S, Büttner P, Expert D, Völksch B, S Ullrich M, et al. Impact of siderophore production by Pseudomonas syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol activity against Pseudomonas syringae pv. glycinea 1a/96. Appl Environ Microbiol. 2010; 76: 2704–2711.
- Matilla MA, Ramos JL, Bakker PA, Doornbos R, Badri DV, Vivanco JM, et al. Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation. See comment in PubMed Commons below Environ Microbiol Rep. 2010; 2: 381-388.
- Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can. J.Micribiol. 1997; 43: 895–914.
- Chernin L, Chet I. Microbial enzymes in biocontrol of plant pathogens and pests. Burns RG, Dick RP, Marcel Dekker, editors. In: Enzymes in the environment: activity, ecology, and applications. New York. 2002: 171–225.
- Krechel A, Faupel A, Hallmann J, Ulrich A, Berg G. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can J Microbiol. 2002; 48: 772-786.
- van Peer R, Punte HL, de Weger LA, Schippers B. Characterization of Root Surface and Endorhizosphere Pseudomonads in Relation to Their Colonization of Roots. Appl Environ Microbiol. 1990; 56: 2462-2470.