
Research Article
Austin J Plant Biol. 2015;1(1): 1004.
Changes in Growth and Some Biochemical Parameters of Maize Plants Irrigated with Sewage Water
Arafat Abdel Hamed Abdel Latef* and Mohammad Mostafa Sallam
Department of Botany, South Valley University, Egypt
*Corresponding author: Arafat Abdel Hamed Abdel Latef, Department of Botany, South Valley University, 83523 Qena, Egypt
Received: February 09, 2015; Accepted: March 04, 2014; Published: March 09, 2015
Abstract
The purpose of this investigation was to study the effect of sewage water irrigation on the growth, photosynthetic pigments, sugars, proteins, total free amino acids, proline and the activity of antioxidant enzymes of maize plants. Results indicated that sewage-irrigated plants in most cases showed a significant increase in growth and all biochemical parameters than the control plants. This increase was generally higher in shoot than root. This study provides evidence for a beneficial effect of sewage water application for maize plants.
Keywords: Antioxidant enzymes; Growth; Maize; Photosynthetic pigments; Proteins; Sewage water; Sugars
Introduction
The wastewater is defined as all water used at homes that goes down the drains or into the wastewater collection system. The use of urban wastewater in agriculture is widely established practice for alleviating water scarcity situations, or even eliminating the purchase of chemical fertilizers. However, unregulated irrigation with untreated wastewater poses serious public health risks through the introduction of metal ions and their accumulation in bio-systems with continuous application to soils [1,2]. These metals could have toxic impact on biological systems if present beyond their respective safe limits in soils, waters and/or plants [3].
Al-Jaloud et al. [4] found that crop yield showed significant increase with increasing water salinity in the wastewater. Their results showed that wastewater can be successfully used to grow maize and sorghum as forage crops. Gadallah [5] reported that sunflower plants treated with sewage wastewater had higher soluble sugars, hydrolysable carbohydrates and soluble protein levels than the control plants (irrigated with tap water), while changes in amino acids content was variable.
Reuse after proper treatment is normally recommended as the main solution of preventing health risks. Unfortunately, because of the high cost of engineering plants, most cities in the developing countries do not have sufficient wastewater treatment capacity.
El-Salhya sewage station was constructed in Qena city, Egypt in 1970. The capacity of this station is 12000m3. Usually, it receives a huge amount of sewage water. The station is unable to filter the impurities of this huge amount of the sewage water efficiently. There is a farm belongs to this station that has an area of 1000 acres of sandy soil. This area is suitable for planting without any reclamation. 20 acres are planted with Eucalyptus and 280 acres with wooden trees. About 700 acres are not planted. There are no heavy industries in Qena city. So the sewage water contains a low or limited concentration of heavy metals.
The aim of the present work was to study the effect of sewage water irrigation on the growth, photosynthetic pigments, sugars, proteins, total free amino acids, proline and antioxidant enzymes activity of maize plants.
Materials and Methods
Experimental material and plant growth
Maize (Zea mays L.) seeds were surface-disinfected by submersing in 1.5% sodium hypochlorite solution for 1-2 min; they were then washed 3 times in sterile distilled water and dried on sterile filter paper in a laminar air flow cabinet. Five seeds were sown in plastic pots containing two kg of air-dried sandy soil. The pots were irrigated regularly with tap water for 7 days then the pots were divided into two groups. The first group was irrigated with tap water as control and the second group was irrigated with raw sewage water collected from El-Salhya sewage station, Qena city, Egypt. The chemical analysis of the sewage water (Table 1) used in irrigation was measured by the method described by Jackson [6]. The treatments were arranged in a complete randomized design with 4 pots for each treatment. After 50 days of irrigation with sewage water, the plants were harvested.
Soluble cations (Μg/L)
Soluble anions (mg/L)
Trace elements (µg/L)
PH
Na+
K+
Ca2+
Mg2+
Cl-
NO3-
SO42-
PO43-
Fe3+
Mn2+
Cu2+
Ni2+
Value
8.1
298.42
55.56
120.09
109.62
130.31
11.32
39.87
27.95
25.31
16.78
10.52
2.98
Table 1: Chemical characteristics of sewage water in El-Salhya station, Qen city, Egypt used in irrigation.
Growth traits
For fresh weight determination, homologous plants were removed, washed, gently blotted, dissected into root and shoot and then the parts were weighed separately. The samples were dried in an oven at 60°C to constant weight to determine the dry weight.
Photosynthetic pigments and metabolite analysis
The photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) contents of youngest fully expanded leaf 1 week before harvest were assayed according to Zhang and Zhang [7]. The extraction was made from a 0.2 g fresh sample in 20 ml ethanol, acetone and water (4.5:4.5:1, v/v/v) mixture and measured at 645, 663, and 470 nm with a UV/VIS spectrophotometer.
Sugars (soluble, insoluble and total) contents were determined by the anthrone sulfuric acid method described by Badour [8]. The dried tissue of root and shoot was extracted by distilled water (in case of soluble sugars) or HCl (in case of total sugars). A 1 ml of the sugar extract was mixed with 9 ml of anthrone sulfuric acid reagent in a test tube and heated for 7 min at 100°C. The absorbance was measured spectrophotometrically at 620 nm against blank containing only distilled water and anthrone reagent.
Protein fractions (soluble, insoluble and total) contents of root and shoot were determined according to the method described by Bradford [9].
Total free amino acids contents were extracted and estimated in root and shoot according to the method of Lee and Takahashi [10]. About 0.1 ml of the water extract containing free amino acids was mixed with 1.9 ml of ninhydrin-citrate-glycerol mixture in a test tube for 20 minutes at 100 °C. The absorbance was measured at 570 nm against a blank (only distilled water and the same reagent).
Proline content of root and shoot was estimated according to Bates et al. [11]. A known weight of dried tissue was homogenized in 10 ml of 3% sulfosalicylic acid and filtered. A 2 ml of the filtrate was made to react with 2 ml glacial acetic acid and 2 ml of acidninhydrin reagent in a test tube and heated for 1 h at 100°C. The reaction mixture was extracted with 4 ml toluene. The chromophore was aspired from the aqueous phase and the absorbance was read at 520 nm using toluene as a blank.
Extraction of antioxidant enzymes
For enzyme extracts and assays, 0.5 g fresh leaves were frozen in liquid nitrogen and then ground in 4 ml solution containing 50 mM phosphate buffer (pH 7.0), 1% (w/v) polyvinylpolypyrrolidone and 0.2 mM ascorbic acid. The homogenate was centrifuged at 15,000 xg for 30 min, and the supernatant was collected for enzyme assays [12].
Assays for antioxidant enzymes activities
The activity of catalase (CAT, EC 1.11.1.6) was determined as a decrease in absorbanceat 240 nm for 1 min following the decomposition of H2O2 [13]. The reaction mixture contained 50 mM phosphate buffer (pH 7.0) and 15 mM H2O2.
Peroxidase (POD, EC 1.11.1.7) activity was measured by following the change of absorption at 470 nm due to guaiacol oxidation. The activity was assayed for 1 min in a reaction solution (3 ml final volume) composed of 100 mM potassium phosphate buffer (pH 7.0), 20 mM guaiacol, 10 mM H2O2 and 0.15 ml enzyme extract [14].
The activity of ascorbate peroxidase (APX, EC 1.11.1.11) was measured as a decrease in absorbance at 290 nm for 1 min [15]. The assay mixture consisted of 0.5 mM ASA, 0.1 mM H2O2, 0.1 mM EDTA, 50 mM sodium phosphate buffer (pH 7.0), and 0.15 ml enzyme extract.
Statistical analysis
All of data were subjected to one-way analysis of variance (ANOVA) using SPSS 12.0 software. Least significant difference (L.S.D) test was used to assess the differences between the mean values of control and sewage-treated plants; p > 0.05 was considered statistically significant.
Plant growth
There are no significant differences between sewage-irrigated plants and control plants on the production of fresh and dry weight of maize root (Figure 1a&1b). However, for maize shoot, the fresh and dry weight significantly increased under sewage water irrigation versus control (Figure 1a&1b).
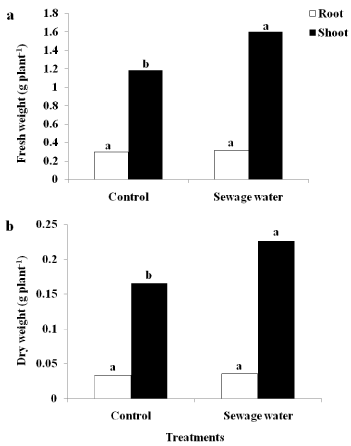
Figure 1: Effect of sewage water irrigation on (a) fresh weight and (b) dry
weight of root and shoot maize plants. Bars carrying different letters are
significantly different at P > 0.05 between the control and sewage treatedplants.
Photosynthetic pigments
In comparison to control, the content of chlorophyll a and b of sewage-irrigated plants markedly increased. This increase was obvious in chlorophyll b than a, but the differences were not significant for carotenoids content (Figure 2).
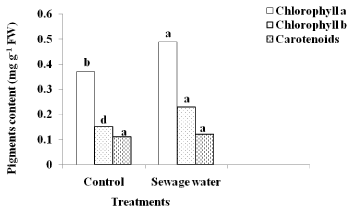
Figure 2: Effect of sewage water irrigation on pigments content of maize
leaves. Bars carrying different letters are significantly different at P > 0.05
between the control and sewage treated-plants.
Sugars
The soluble sugar content of maize root was higher in sewageirrigated plants than control plants, but the content of insoluble and total sugars remained unchanged as a result of sewage water irrigation (Figure 3a). In maize shoot, sewage water treatment improved the contents of soluble, insoluble and total sugars in comparison to control (Figure 3b).
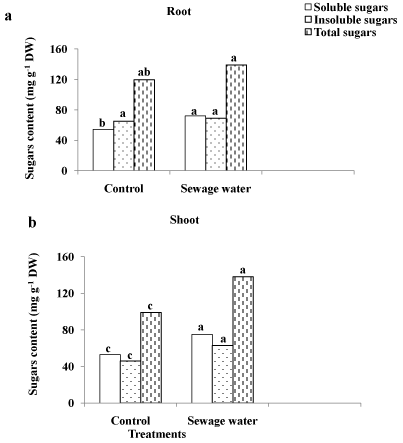
Figure 3: Effect of sewage water irrigation on sugars content of (a) root
and (b) shoot of maize plants. Bars carrying different letters are significantly
different at P > 0.05 between the control and sewage treated-plants.
Proteins
In maize root, there are no significant differences in the content of proteins (soluble, insoluble and total) between sewage-irrigated plants and control plants (Figure 4a). In maize shoot, sewage water irrigation resulted in an accumulation of all protein fractions contents as compared to control (Figure 4b).
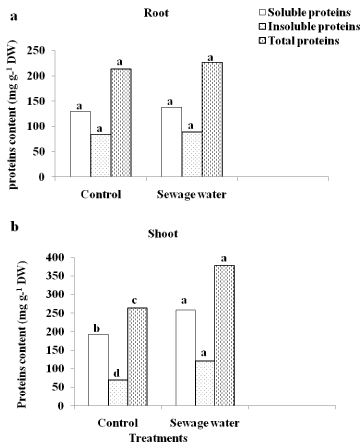
Figure 4: Effect of sewage water irrigation on proteins content of (a) root
and (b) shoot of maize plants. Bars carrying different letters are significantly
different at P > 0.05 between the control and sewage treated-plants.
Total free amino acids and proline
It was shown that the sewage water irrigation enhanced the accumulation of total free amino acids (Figure 5a) and proline contents (Figure 5b) in both root and shoot maize compared to the control plants. This accumulation was noticed in shoot than root.
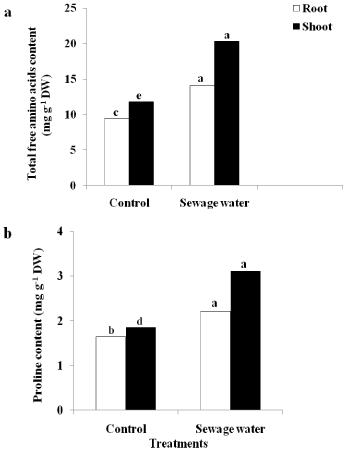
Figure 5: Effect of sewage water irrigation on (a) total free amino acids
content and (b) proline content of root and shoot of maize plants. Bars
carrying different letters are significantly different at P > 0.05 between the
control and sewage treated-plants.
Activities of antioxidant enzymes
Compared to control, sewage water irrigation improved the activities of CAT, POD and APX in maize leaves. This improvement was 65%, 37% and 56% in CAT, POD and APX activity respectively over the control (Figure 6).
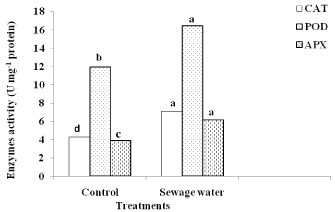
Figure 6: Effect of sewage water irrigation on enzymes activity of maize
leaves. Bars carrying different letters are significantly different at P > 0.05
between the control and sewage treated-plants.
Discussion
The data presented in Table 1 indicated that the cations content of sewage water were arranged in the following descending order: Na+<
Ca2+< Mg2+< K+. The anions content were arranged in the following
descending order. Cl-
In the present study, The increase in growth (especially in maize shoot) as a result of sewage water irrigation may be due to the increase in organic matter, macro-and micronutrients in the sewage water where beneficial nutrients improved the metabolic activities and hence the vegetative growth [17].
Application of sewage water positively affected synthesis of photosynthetic pigments. The stimulatory effect of sewage water on chlorophyll a and b content could be attributed to the fact that sewage water enhances the rate of biosynthesis of chlorophyll a and b. The increased content of chlorophyll was in parallel with the enhancement of maize shoot growth. i.e, there is an intimate relationship between shoot growth and chlorophyll content [18]. El-Maghraby and Gomaa [19] reported that sewage water application increased number of green leaves and leaf area per plants or it may be increase both of macro and micronutrients elements in soil, which is essential for the plant growth and photosynthetic pigments.
Our results indicated that, sewage water irrigation increased in most cases the content of sugars in maize plants. This increase may be due to the presence of some mineral ions e.g. Mn and Cu that stimulate the two photosystems. Mn2+ is required for PSII (O2 evolving system) and there is also a direct interaction between copper and ferredoxin on the reducing site of PSI. Cu2+ stimulates the rate of overall electron transfer from water to NADP [20]. The data of nitrogen metabolism indicated that the irrigation of the experimental plants with sewage water induced mostly a pronounced accumulation of proteins, total free amino acids and proline in maize root and shoot. The accumulation in nitrogen compounds may be due to presence of soluble or organic or inorganic substances in the sewage water which may generate higher growth. A significant increase in the biochemical constituents like protein and amino acid contents in the fodder grass irrigated with sewage water during summer season was reported by Girisha et al. [21]. Similar results were reported by Rija et al [22] in their study of sewage irrigation on plants like Vigna radiata, Cicer arietinum and Lens culinaris. They recorded an increase in total protein, carbohydrate and chlorophyll contents in L. culinaris and C. arietinum leaf samples. Rija et al. [22] reported that the increase in biochemical parameters such as chlorophyll, carbohydrates and protein may be also due to the activity of some microbes present in sewage water which can convert organic matter into by-products like CO2, NO3, PO4, SO4, NH3, H2S and CH4 [23,24]. Also, presence of higher amounts of macronutrients in the sewage water provides cofactors to enzymes necessary for the synthesis of protein and carbohydrate. Besides, high proline content measured in sewage water plants could also contribute to a protective role as scavenger of Reactive Oxygen Species (ROS) [25].
ROS scavenging can be achieved by antioxidant enzymes like CAT, POD and APX. In this work, sewage water treatment resulted in an increase in the activities of CAT, POD and APX in leaves of maize plants which can be a manifestation of the initiation of antioxidant defense and might be a part of adaptive strategy of maize irrigated with sewage water.
In conclusion, based on the measured growth parameters, sewage water of El-Salhya station, Qena city, Egypt could successfully be used for irrigation of maize plants grown in sandy soils. This is because (1) it contains a low or limited concentration of heavy metals, (2) its positive effects on their growth and nutritive values. However, further research involving different plants is needed.
References
- Abdel-Saheb I, Schwab AP, Banks MK, Hetrick BA. Chemical characterization of heavy metal contaminated soil in south-east Kansas. Water Air Soil Pollut. 1994; 78: 73-82.
- Wallace GA, Wallace A. Lead and other potentially toxic heavy metals in soil. Commun Soil Sci Plant Analy. 1994; 25: 137-141.
- Hussain SI, Ghafoor A, Ahmad S, Murtaza G, Sabir M. Irrigation of crops with raw sewage: Hazard assessment of effluent, soil and vegetables. Pak J AgriSci. 2006; 43: 97-102.
- Al-Jaloud AA, Ghulam H, Al-Saati AJ, Sheikh K. Effect of waste-water on plant growth and soil properties. Arid Soil Res. 1993; 7: 173–179.
- Gadallah MA. Effects of industrial and sewage wastewater on the concentration of the soluble carbon, nitrogen and some minerals elements in sunflower plants. J Plant Nut. 1994; 17: 1369-1384.
- Jackson ML. Soil Chemical Analysis. Prentice Hall, Inc. Englewood Cliffs NJ. Iobary of Congress, M.A.S. 1967.
- Zhang ZA, Zhang MS. Experimental guide for plant physiology. High education, Beijing. 2006
- Badour SSA. Analytisch-chemische Untersuchung des Kaliummangels bei Chlorella im Vergleich mit anderen Mangelzuständen. Ph.D. Dissertation Göttingen. [Analytical-chemical investigation of potassium de?ciency in Chlorellain comparison with other de?ciencies]. Ph.D. Dissertation, Göttingen University, Göttingen, Germany. 1959.
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem.1976; 72: 248–254.
- Lee YP, Takanashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem.1966; 14: 71–77.
- Bates LS, Wladren P, Tear DT. Rapid determination of free proline for water-stress studies. Plant Soil. 1973; 39: 205–207.
- Abdel Latef AA. Influence of arbuscularmycorrhizal fungi and copper on growth, accumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuumL). Mycorrhiza. 2011; 21: 495-503.
- Chance B, Maehly CA. Assay of catalases and peroxidase. Methods Enzymol. 1955; 2: 764-775.
- Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of Norway (PiceaabiesL.). Plant Physiol. 1994; 106: 53–60.
- NakanoY, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981; 22: 867–880.
- Hussein AA. Studies on the significance of algal control of sewage water and its resue in the irrigation of some economical plants. Ph.D. Thesis, South Valley University, Qena, Egypt. 2008; 1-183.
- El-Maghraby SS, Gomaa MA. Effect of sewage sludge as a fertilizer on syrup yieldand some agronomic characters of sweet sorghum. Egypt J Applied Sci. 1992; 7: 300-317.
- Zeid IM, Abou El Ghate HM. Effect of sewage water on growth, metabolism and yield of bean. J Biol Sci. 2007; 7: 34-40.
- EL-Sawaf N. Response of Sorghum spp. to sewage waste-water irrigation. Int J Agri Biol. 2005; 6: 869-874.
- Mazen A, Faheed FA, Ahmed AF. Study of potential impacts of using sewage sludge in the amendment of desert reclaimed soil on wheat and jews mallow plants. Braz Arch Biol Technol. 2010; 53: 917-930.
- Girisha ST, Muniyamma M, Umesh S. Impact of sewage water on protein, carbohydrate and amino acid contents of the fodder grass, Brachilariamutica. J Eco Biol. 2007; 21: 241-246.
- Rija H, Siddigui ZS, Zaman AU. Changes in chlorophyll, protein and carbohydrate contents of Vigna radiate, Cicerarietinum and Lens culinaris after irrigation with sewage water. Int j Biol Biotech. 2005; 2: 981-984.
- Almazov BN, Kholuyako LT. Change in productivity of a vegetable crop rotation and fertility of leachedchemozem soil in relation to application of organic manures and mineral fertilizers. Agrokhimiya. 1990; 1: 53-60.
- Baghel MS, Singh DB. Effect of different levels of nitrogen, potash and dates of transplanting on cauliflower. Recent Hort. 1995; 2: 84-87.
- Antolín MC, Muro I, Sánchez-Díaz M. Application of sewage sludge improves growth, photosynthesis and antioxidant activities of nodulated alfalfa plants under drought conditions. Environ Exp Bot. 2010; 68:75–82.