
Research Article
Austin J Plant Biol. 2015;1(1): 1005.
Correlative Influence of Axes on Senescence of Cotyledons Following Germination of Mung Bean Vigna Radiata (L.) Wilczek Seeds
Pal L and Kar RK*
Department of Botany, Visva Bharati University, India
*Corresponding author: Kar RK, Plant Physiology and Biochemistry Laboratory, Department of Botany, Visva Bharati University, Santiniketan 731235, West Bengal, India
Received: May 05, 2015; Accepted: June 24, 2015; Published: June 30, 2015
Abstract
Immediately after seed germination cotyledons undergo transient development culminating in senescence, which is different from that of a leaf. In the present study senescence of mung bean (Vigna radiata) cotyledons following germination has been characterized to understand correlative influence of axes. Cotyledons isolated from axes showed retardation of storage protein degradation as well as increased chlorophyll synthesis followed by senescenceinduced decline. When one of the pair of cotyledons was detached from the axis, the attached cotyledon showed same characters like intact cotyledons but the detached one showed the behavior similar to the isolated cotyledons. When epicotyls were removed after 3 days incubation cotyledons remained attached with axes for longer period and decline in chlorophyll content retarded compared to control, but decline in protein level remained unaffected. Removal of root tip marginally delayed the decline in both chlorophyll and protein level. Morphological studies also showed maintenance of greenness and fullness of cotyledons when isolated or epicotyls removed. Histological studies revealed that storage bodies started degenerating following germination from the peripheral zone while chloroplast development progressed around vascular bundle ending with signs of senescence in the last phase. Both storage body degeneration and chloroplastic senescence was delayed in case of isolated cotyledons. Therefore, cotyledons in case of epigeous germination of seeds pass through complex developmental changes. Immediately after germination storage mobilization starts from the periphery to provide soluble substrates to growing axes along with photosynthetic development around vascular bundle; the latter ends with senescence-induced degeneration influenced by emerging epicotyl acting possibly as sink.
Keywords: Chlorophyll; cotyledon senescence; epicotyl decapitation; protein; root tip removal
Introduction
Senescence is a programmed developmental process that occurs at every stage of plant’s life cycle. Thus following seed germination seedling development starts with cotyledon(s) undergoing transient development along with the growth of embryonic axes followed by senescence in case of most dicotyledonous seeds. Indeed cotyledons have a short life span, at the end of which these finally shrink and detached from the axes with the appearance of differentiated leaves [1]. The major physiological function of cotyledons is to provide substrates through storage mobilization for the development of the growing seedling until differentiation of photosynthetically efficient leaves. In dicot seeds, particularly non-endospermic legume seeds storage proteins remain sequestered compactly in protein storage vacuoles of parenchyma cells of cotyledons [2] and during germination such proteins undergo hydrolysis by proteinases resulting in production of amino acids that mobilizes to the embryonic axes to support growth [3]. During this period, cotyledons gradually become green in colour and plastids develop into functional chloroplasts [4] before these finally senesce and detached from axes. Fundamentally senescence process in leaves and cotyledons may be same, but as a special organ cotyledons are likely to have a differently regulated senescence under the correlative influence of growing embryonic axes during germination.In the present study attempt has been made to characterize cotyledon senescence of Vigna radiata seeds in the context of correlative influence of growing axes following germination by monitoring chlorophyll and protein levels and morphological and histological studies of cotyledons either in isolation or under removal of epicotyl or root tip.
Materials and Methods
Incubation
Seeds of Vigna radiata (L.) Wilczek (var. B1) were collected from Pulses and Oilseeds Research Station, Berhampur, Murshidabad, West Bengal. Healthy seeds were first surface sterilized with 1% sodium hypochlorite solution for 5 minutes, rinsed in distilled water several times and dried on tissue paper. Seeds were then placed in covered plastic Petri dishes (9 cm) on Whatman no.1 filter paper soaked in distilled water. These sets were then incubated in darkness under controlled temperature (30±2°C) in a seed germinator. After 24 h germination, germinated seeds or isolated cotyledons were incubated either in light or in darkness for different durations and at intervals cotyledons were analysed for chlorophyll and protein contents. Cotyledons from seedlings with root tip removed or decapitated epicotyl were also used for similar assessments. Assessments for chlorophyll and protein contents were done at 2 day intervals in most of the cases except in case of comparative assessment between attached and detached cotyledons and in case of root tip removed cotyledons where samples were collected at 1 day intervals.
Chlorophyll estimation
Cotyledons (5 pairs in number) were washed with distilled water and the surface water was soaked with tissue paper. These were then homogenized with 5 ml 80% acetone and centrifuged at 5000×g for 10 minutes. The supernatants were decanted and made up to a definite volume (5 ml). The contents of chlorophyll a and chlorophyll b separately as well as total chlorophyll content were estimated by recording the absorbance at 663nm and 645nm in a spectrophotometer following the method of Arnon [5]. The chlorophyll content was expressed as μg/pair of cotyledons.
Protein estimation
For the determination of the protein content, the residues (pellet) of the samples, from which the chlorophyll was removed, were digested in 2 ml of 1(N) NaOH for 1 h at 800C. After digestion the extract was diluted appropriately with distilled water. To 1 ml of this final (diluted) extract, 1 ml of the mixture of Reagent a 10% sodium carbonate in 0.5 (N) NaOH, 1% copper sulphate and 2% sodium potassium tartarate in the ratio of 20:1:1 was added. After 5 min Folin Phenol Reagent (Reagent B), diluted to 1:2 with water, was added to the mixture and the protein content was estimated by measuring the absorbance of the blue colour developed after the reaction at 650nm in a spectrophotometer according to the method of Lowry et al. [6]. A standard curve was prepared using Bovine Serum Albumin (BSA). The protein content was expressed as mg/pair of cotyledons.
Morphology and histological studies of cotyledons
Morphology of cotyledons for seedlings subjected to epicotyl decapitation, root tip removal, cotyledons isolated from or left attached to axes was studied and photographed. Anatomy of cotyledons was studied by observing transverse sections of cotyledons, either intact or isolated, under light microscope at different intervals during incubation in light. Starch grains and protein body distribution were determined by staining freehand sections of cotyledons with iodine and toluidine blue, respectively. All the sections used in these experiments were obtained by cutting single cotyledons transversely. Live sections were stained with 0.01% toluidine blue in 0.1% aqueous sodium tetraborate (for protein bodies) and 0.5% iodine in 5% aqueous potassium iodide (for starch grains) for light microscope observations.
Results
It was observed that the intact cotyledons remained attached with the germinating axis until day 5 after which they were detached from the axis. Total chlorophyll content of these cotyledons incubated in light increased from day 1 up to day 3 followed by a decrease. On the other hand, isolated cotyledons were maintained up to day 13 and the chlorophyll content of cotyledons constantly increased from day 1 to day 7 reaching a value almost 5 times of the maximum level recorded in intact cotyledons and then decreased gradually thereafter up to day 13 under light (Figure 1c). Although the content of chlorophyll a of the cotyledons (both intact and isolated) was far more than that of chlorophyll b, the trend of changes with incubation was same for both chlorophyll a and chlorophyll b contents (Figures 1a and 1b). There was negligible chlorophyll production in dark conditions. As there was no significant chlorophyll production noted in dark in case of subsequent experiments, no data were presented for chlorophyll level changes in cotyledons from seeds incubated in dark. But protein content of cotyledons was checked in both light and dark conditions to ascertain any difference in protein level changes, mainly due to storage mobilization in case of light-incubated and dark-incubated seedlings.However, the protein content was found to decrease drastically from day 1 to day 5 to a minimum level in intact cotyledons similarly under both light and dark condition, whereas the protein content was maintained more or less at the same level under both light and dark incubation in isolated cotyledons after an initial fall at day 3 (Figure 1d).
In case of another experiment, where one cotyledon of the pair of cotyledons from an intact seedling was detached while other one left attached with axis, cotyledons were analysed for contents of total chlorophyll and chlorophyll a and b separately incubating seeds in light only and protein content during incubation both in light and darkness (Figures 2 and 3). Both the contents of chlorophyll (a and b and total) (Figures 2a, b and c) and protein (Figures 3a and b) of the cotyledons which were attached with the germinating axis, declined similar to the intact cotyledons i.e. an initial increase up to day 3 followed by decline in case of chlorophyll a and b and a gradual decline from the beginning for protein content both in light (Figure 3a) and dark (Figure 3b). On the other hand, the detached cotyledons (without axes) showed the characters like isolated cotyledons i.e. gradual increase in the contents of chlorophyll a and b (up to day 5) and retarded decline of protein level.
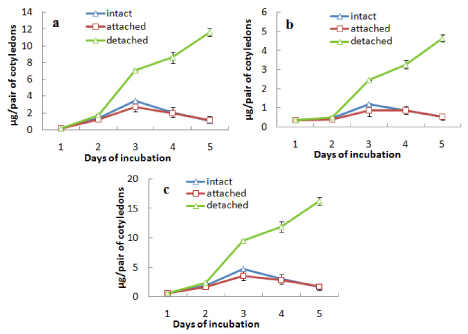
Figure 2: Changes in the contents of (a) chlorophyll a, (b) chlorophyll b and
(c) total chlorophyll in attached and detached cotyledons along with those
from an intact seedling during seedling growth in light ± SE shown as vertical
bars.
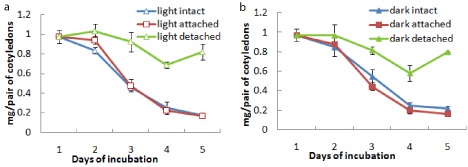
Figure 3: Changes in the contents of protein in attached and detached
cotyledons along with those from an intact seedling during seedling growth in
(a) light and (b) dark ± SE shown as vertical bars.
When epicotyl portion was decapitated after day 3 the attachment of cotyledons with germinating axis was extended up to day 9. During this period the content of chlorophylls (a, b and total) was maintained or slightly increased after decapitation up to day 7 and then decreased rapidly (Figures 4a, b and c). The protein content of the cotyledons from both light- and dark-incubated seedlings where epicotyls were decapitated showed the characters similar to intact cotyledons, i.e. in all cases protein content declined sharply from the beginning and maintained thereafter at a minimal level (Figure 4d). In another surgical experiment, root tips were removed from the seedlings after day 3 and cotyledons were analysed for the contents of chlorophylls (a and b) and protein content (Figure 5). It was observed that the content of chlorophylls (a, b and total) was slightly maintained for a day (from day 3 to day 4) in case of cotyledons from seedlings where root tips were removed but finally declined to the level of control at the end of 5 d incubation (Figures 5a-5c). Protein contents of cotyledons from seedlings, where root tips were removed, also showed a slight retardation of decline rate compared to control both during light and dark incubation (Figure 5d). Morphology of cotyledons from seedlings manipulated differently showed that the cotyledons looked more green, less shrinked and little large in case of cotyledons from seedlings where epicotyls decapitated, cotyledon isolated, root tip removed compared to control (attached) seedlings (Figure 6). Histological studies showed that the storage cells of cotyledons were fully packed with starch grains and protein bodies immediately after the germination of seeds (Figures 7-10). In later days breakdown or lysis of storage bodies started in the cells located farthest from the vascular bundles throughout the cotyledons. In case of intact cotyledons, progress of such degeneration of starch grains and protein bodies was faster (Figures 7 and 8) than in case of isolated cotyledons (Figures 9 and 10). Besides storage bodies, green patches containing chlorophyllous structures gradually developed around vascular bundles during incubation and again such development occurred faster in case of intact cotyledons than in isolated cotyledons. However, in latter case such green chlorophyllous patches were maintained for longer (up to day 7). A closer view (Figures 11a-11f) of protein bodies, starch grains reveals their structure and distribution in cotyledons cells using stain (toluidine blue for protein bodies and iodine for starch grains). Also chlorophyllous patches around vascular bundles show developing chloroplasts (Figure 11b).
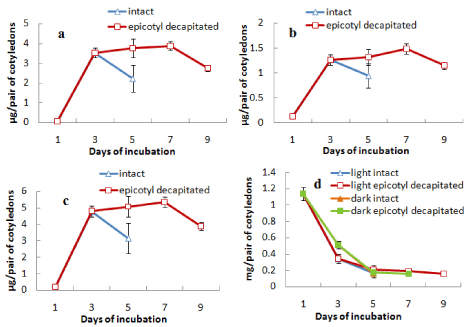
Figure 4: Changes in the contents of (a) chlorophyll a, (b) chlorophyll b,
(c) total chlorophyll and (d) protein in cotyledons from intact seedlings or
seedlings having epicotyl decapitated (after 3 days) during seedling growth
either in light only (in case of chlorophyll content) or both in light and dark (in
case or protein content) ± SE shown as vertical bars.
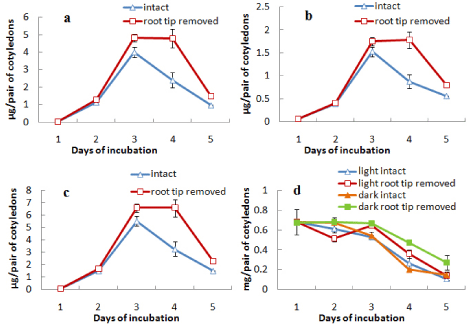
Figure 5: Changes in the contents of (a) chlorophyll a, (b) chlorophyll b,
(c) total chlorophyll and (d) protein in cotyledons from intact seedlings or
seedlings having root tip removed (after 3 days) during seedling growth either
in light only (in case of chlorophyll content) or both in light and dark (in case
or protein content) ± SE shown as vertical bars.

Figure 6: Morphology of cotyledons in case of (a) intact seedling (day 4), (b)
seedling having epicotyl decapitated (day 4), (c) attached with axis (day 3),
(d) seedling having root tip removed (day 3) and (e) isolated from seedling
(day 5).
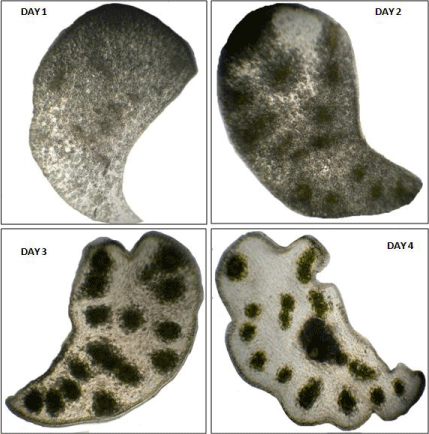
Figure 7: Transverse sections of cotyledons from intact seedlings incubated
in light for different days (day 1, day 2, day 3, day 4) as observed under low
power objective (10X) of light microscope.
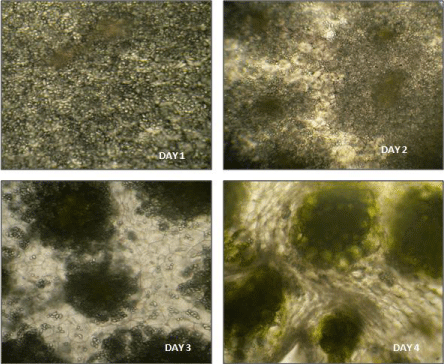
Figure 8: Transverse sections of cotyledons from intact seedlings incubated
in light for different days (day 1, day 2, day 3, day 4) as observed under high
power objective (40X) of light microscope.

Figure 9: Transverse sections of cotyledons isolated from seedlings
incubated in light for different days (day 1, day 3, day 5, day 7) as observed
under low power objective (10X) of light microscope.
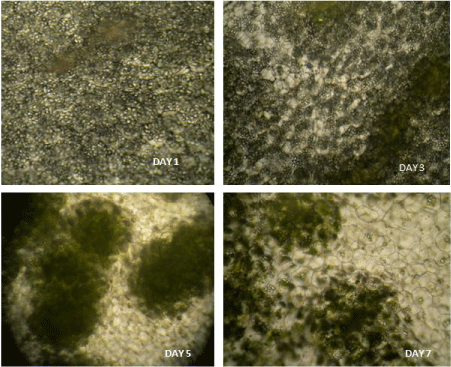
Figure 10: Transverse sections of cotyledons isolated from seedlings
incubated in light for different days (day 1, day 3, day 5, day 7) as observed
under high power objective (40X) of light microscope.
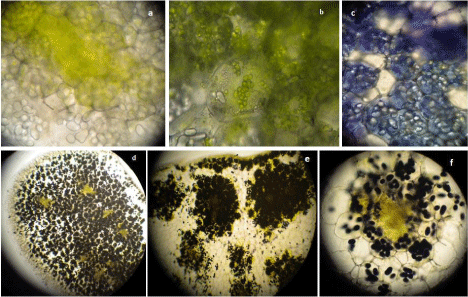
Figure 11: Transverse sections of intact cotyledons showing (a) vascular
bundle region with chlorophyllous patch, (b) chloroplasts in the storage
cells, (c) toluidine stained protein bodies and iodine stained starch grains as
observed under (d) 5X, (e) 10X, (f) 40X objectives of light microscope.
Discussion
Senescence may be considered as the final stage in the differentiation of an organ. The cotyledons attached with an intact axis undergo this last phase of differentiation shortly after seed germination. In case of dicot seeds, particularly non-endospermic seeds of Vigna radiata that germinate epigeously, cotyledons undergo autolysis of storage bodies like starch grains and protein bodies along with a parallel photosynthetic development, which is transient ending with degeneration of cotyledons. Thus degradation of storage proteins (and also starch) within lytic vacuoles starts immediately following germination while synthetic processes characteristics of plastidial development occurs at the same time in the cotyledons. However, our histological studies showed that storage breakdown started from the peripheral region of cotyledons while chlorophyllous patches, probably related to chloroplast development, appeared around the vascular bundles. Similar observations have also been made in Vigna radiata seeds by Harris and Chrispeels [7] and in Vigna mungo by Toyooka et al. [8]. They have pointed out that such storage protein bodies undergo autolysis which is regulated during germination. Further studies with cotyledons of Vigna mungo seeds showed that the storage proteins in the protein bodies or Protein Storage Vacuoles (PSV) are degraded by proteinases converting PSV to a lytic vacuole [9,10]. Again, in case of V. mungo, two distinct autophagic mechanisms have been proposed for degradation of starch grains and cellular components [11]. However, degradation of storage proteins is clearly under control of embryonic axes since protein level was mostly maintained rather somewhat increased after initial fall up to day 3 in case of isolated cotyledons in contrast to intact cotyledons where the protein level fell to a minimum level at the end 5 days incubation (Figure 1). Experiment with one of the cotyledons detached while the other left attached followed by their analysis also reflects the same (Figures 2 & 3). Interestingly, these changes in protein level were similar under light as well as darkness during incubation, whereas chlorophyll synthesis and putative chloroplast development occurs only in light-incubated cotyledons. Therefore it appears that early photosynthetic development of cotyledons in light grown seedlings is not related with storage protein degradation. However, in light incubated seedlings chlorophyll level increased almost five times in case of isolated cotyledons over the content of cotyledons from intact seedlings, thus deferring the senescence-induced decline further up to day 7. This indicates that chlorophyll synthesis during cotyledon development followed by degradation during senescence is also influenced by the embryonic axes. Chloroplast development programme in cotyledons and true leaf is differently regulated. Two genes, CYO1 and SCO3, have been identified for their role in cotyledon-specific chloroplast development [4,12].
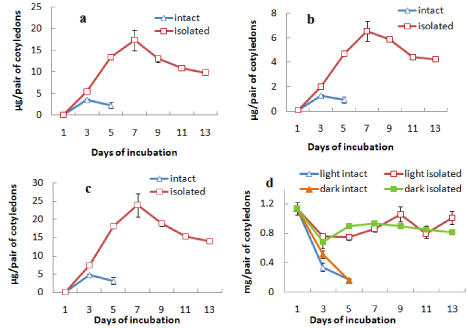
Figure 1: Changes in the contents of (a) chlorophyll a, (b) chlorophyll b,
(c) total chlorophyll and (d) protein in intact and isolated cotyledons during
seedling growth either in light only (in case of chlorophyll content) or both in
light and dark (in case or protein content) ± SE shown as vertical bars.
As a case of correlative influence, some kind of signal may be hormonal is likely to come from axes including both shoot tip and root tip that ultimately influences the developmental changes in cotyledons during seed germination. When the growing epicotyls of light grown seedlings of V. radiata were decapitated, chlorophyll synthetic phase of cotyledons was prolonged thus deferring the senescence-induced decline (up to day 7), but protein degradation was unaffected (Figure 4). Decapitation usually leads to metabolic changes and transport of plant hormones, particularly auxin and cytokinins. Wilhelmová et al. [13] have demonstrated an increase in cytokinin level in bean cotyledons from seedlings having epicotyl decapitated. Based on the recent knowledge on interactive role of auxin and cytokinin in case of correlative dominance, delay of cotyledon senescence due to decapitation may be explained by the elevated cytokinin level because of de novo synthesis in the nodal stem along with elimination of competition from the epicotyl as a sink for cytokinins [14]. Also, decapitation of epicotyl may preserve PSII electron transport activity that can slow down the process of senescence of cotyledons in the decapitated plants [15]. Another change due to epicotyl detachment was the prolonged attachment of cotyledons with axes, i.e. delay in abscission of cotyledons, which may be a result of changed auxin gradient due to elimination of shoot apex-originated auxin. However, somewhat delay of decline in chlorophyll level in cotyledons while root tip was removed (Figure 5) cannot be explained by similar changes in cytokinins. Moreover, here root tip removal also slightly slowed down the decline in protein level of cotyledons. This possibly suggests for involvement of some other kind of root tip-derived signaling. Morphological studies of the cotyledons (Figure 6) also reveal the physical appearance of cotyledons which look full not shrunk much and greener when isolated or detached, epicotyls decapitated or root tip removed. Histological preparations of cotyledons either detached from or attached with seedlings clearly indicate about two zonesperipheral zone where storage mobilization protein degradation in protein bodies and starch degradation in starch grains proceeds from periphery and Vascular Bundle (VB) associated zone where chloroplast development revealed by chlorophyll synthesis occurs ending with a senescence-associated decline. In isolated condition these processes are delayed for the lack of signal from axes. Indeed, cotyledons in case of greening seedlings epigeous germination pass through complex developmental changes. Immediately after germination radicle emergence storage mobilization starts providing soluble substrates as a source to the elongating axes along with chloroplast development in a zone spatially separated (VB zone) that may act as a local sink. In the next phase, when most of the storage is exhausted, chlorophyllous zone (VB zone) undergoes senescence under the influence of epicotyl, former acting as a source while the latter as a sink now. The exact role of vascular bundle in either delaying the storage mobilization or supporting photosynthetic development in the vicinity is not clear at the moment. Moreover, photosynthetic contribution of the greening cotyledons of Vigna radiata that undergo a transient photosynthetic development is questionable.
Acknowledgment
One of the authors (LP) gratefully acknowledges the financial support from University Grants Commission, New Delhi, India in the form of BSR Fellowship.
References
- Marshall PE, Kozlowski TT. Importance of photosynthetic cotyledons for early growth of woody angiosperms. Physiol Plant. 1976; 37: 336–340.
- Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, et al. The protein storage vacuole: a unique compound organelle. J Cell Biol. 2001; 155: 991-1002.
- Bewley, JD, Black M. Seeds: Physiology of Development and Germination. 2nd edn. New York: Plenum Press. 1994.
- Shimada H, Mochizuki M, Ogura K, Froehlich JE, Osteryoung KW, Shirano Y, et al. Arabidopsis cotyledon-specific chloroplast biogenesis factor CYO1 is a protein disulfide isomerase. Plant Cell. 2007; 19: 3157-3169.
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949; 24: 1-15.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
- Harris N, Chrispeels MJ. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975; 56: 292-299.
- Toyooka K, Okamoto T, Minamikawa T. Mass transport of a proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J Cell Biol. 2000; 148: 453–464.
- Mitsuhashi W, Koshiba T, Minamikawa T. Separation and Characterization of Two Endopeptidases from Cotyledons of Germinating Vigna mungo Seeds. Plant Physiol. 1986; 80: 628-634.
- Okamoto T, Minamikawa T. A vacuolar cysteine endopeptidase (SHEP) that digests seed storage globulin: characterization, regulation of gene expression, and posttranslational processing. J Plant Physiol. 1998; 152: 675–682.
- Toyooka K, Okamoto T, Minamikawa T. Cotyledon cells of Vigna mungo seedlings use at least two distinct autophagic machineries for degradation of starch granules and cellular components. J Cell Biol. 2001; 154: 973-982.
- Albrecht V, Simková K, Carrie C, Delannoy E, Giraud E, Whelan J, et al. The cytoskeleton and the peroxisomal-targeted snowy cotyledon3 protein are required for chloroplast development in Arabidopsis. Plant Cell. 2010; 22: 3423-3438.
- Wilhelmová N, Procházkova D, Machácková I, Vágner M, Srbová M, Wilhelm J. The role of cytokinins and ethylene in bean cotyledon senescence. The effect of free radicals. Biol Plant. 2004; 48: 523-529.
- Stefanov D, Ananieva K, Yordanov I. Decapitation and defoliation as approaches to study control mechanisms of leaf senescence and its reversibility. Genetics Plant Physiol. 2011; 1: 3–30.
- Yordanov I, Goltsev V, Stefanov D, Chernev P, Zaharieva I, Kirova M, et al. Preservation of photosynthetic electron transport from senescence-induced inactivation in primary leaves after decapitation and defoliation of bean plants. J Plant Physiol. 2008; 165: 1954–1963.