
Research Article
Austin J Plant Biol. 2022; 8(1): 1031.
Phytotoxicity of Fungicide Coated Sugar Beet Seed Depends on Growth Condition
Haque ME1 and Parvin MS2,3*
¹Department of Plant Pathology, North Dakota State University, Fargo 58108, USA
²Leibniz University Hannover, Germany
³Bangladesh Agricultural Research Institute, Joydebpur, Gazipur, Bangladesh
*Corresponding author: Most Shanaj Parvin, Leibniz University Hannover, Germany; Bangladesh Agricultural Research Institute, Joydebpur, Gazipur, Bangladesh
Received: December 31, 2021; Accepted: January 25, 2022; Published: February 01, 2022
Abstract
Fungicide-coated seed protects sugar beet plants from soilborne diseases, but seedlings coming from coated seeds often encounter phytotoxicity under field conditions. To understand the phytotoxic impact, fungicide-coated seed and the uncoated seed of two cultivars were sown with holes or no holes in plastic trays in greenhouse conditions. Our study demonstrated without fungicide coat on sugar beet seed and holes in plastic trays resulted in just above 90% germination. While fungicide-coated seed and no hole’s underneath traysshowed the lowest germination (>20%). Fungicide-coated seed, having holes in plastic trays showed 90% germination. No fungicide coat on seed, having no hole’s underneath trays showed 70% germination. We further estimated the percentage of stunted seedlings in both cultivars. Fungicide-coated seed with holes underneath plastic trays showed above 5% stunted seedlings while fungicide-coated seed, having no hole’s underneath trays- showed the highest percentage of stunted seedlings (>10%) in both cultivars. In summary, our data demonstrated that the phytotoxicity of fungicide-coated sugar beet seed depends on growth conditions.
Keywords: Fungicide-coated; Sugar beet seed; Phytotoxicity
Introduction
Fungicides are considered one of the most effective control measures for mitigating fungal diseases [1]. Several studies demonstrated that fungicides boost plant defenses through phytoalexin synthesis and cell wall lignification or stimulate enzymes involved in the synthesis of phenolic compounds [2]. However, fungicide toxicity is not always restricted to the eukaryotic heterotrophs (fungi), but also in eukaryotic autotrophs (Plants) [3,4].
Fungicides often cause plant injury or phytotoxicity when a fungicide or insecticide is applied directly to the plant during inclement weather. This can also occur when a chemical dose or rate is not applied properly.
There are several phenotypes that reflect phytotoxicity such as poor germination, especially if a soil drench was used, immature death of seedlings, sudden death of rapidly growing succulent tissues, stunting or delayed seed germination and plant development, misshaped or distorted plants, fruits, or leaves, russeting or bronzing of leaves or fruit, and dead spots or flecks on leaves [5-8]. Phytotoxicity generally recovers with age, but not always.
Several lines of evidence suggest that difenoconazole exposure inhibited shoot growth and leaf area reduce in root dry weight, total root length, and surface area under different regimes of treatment concentrations and periods. Studies further demonstrated that difenoconazole exposure induce activities of superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (G-POD), and ascorbate peroxidase (APX) in the roots and leaves of the wheat seedlings [9]. Other evidences on Triazole coated maize seed controlling soilborne diseases, but seedlings shown phytotoxicity under chilling stress [10].
Another research demonstrated that high temperatures and humidity increase the possibility of injury from insecticides and fungicides. These negatively effects on crop physiology, especially on photosynthesis [11]. Other research on root application of ammonium salts, most notably trinbutyl and trinpentyl-4- chlorobenzylammonium bromides, to wheat seeding grown in water culture enhanced resistance to powdery mildew (Erysiphe graminis) but not to brown rust (Puccinia recondita). When the same chemicals were applied to sand or soil-grown seedlings, this showed less antimildew activity [5].
Sugar beet seed use in North Dakota and Minnesota are primarily treated with fungicides included Kabina, Systiva, Vibrance, and Metlock Suite, individually or in combinations. These treated seed are effective in protecting the seedlings for up to 4-5 weeks and any activity afterwards will depend on factors such as soil conditions and microbial activity [12].
This present study evaluated how phytotoxicity of fungicide coated sugar beet seed depends on growth condition in the greenhouse. In order to understand the phytotoxic impact, fungicide-coated seed and the uncoated seed of two cultivars were sown in plastic trays with holes or no holes. Fungicide-coated seed and no hole’s underneath trays- showed the lowest germination (>20%) and the same growth condition showed the highest percentage of stunted seedlings (>10%) in both cultivars.
Materials and Methods
Greenhouse evaluation of seed germination under different growth conditions
In order to evaluate the germination potential of two sugar beet cultivars, a greenhouse study was done. Fungicide coat was removed with running water and dried for 10 minutes-to develop uncoated seed (Figure 1). Two different types of trays were used, such as; holes in the bottom and without any holes. Four growth conditions were applied to Beta 336 and Beta 4731 as follows: (1) No fungicide coat on sugar beet seed, hole underneath plastic trays; (2) No fungicide coat on seed, no hole underneath plastic trays; (3) fungicide coated seed, hole underneath plastic trays, and (4) fungicide coated seed, no hole underneath plastic trays. Plastic trays (10.94” W x 21.44” L x 2.44” D, 1020 Trays, Heavy Duty, Greenhouse megastore) were filled with vermiculite and perlite mixer (PRO-MIX FLX) amended with osmocote (N-P-K:15-9-12) fertilizer (Scotts Company; Marysville, OH). Twenty seeds of each cultivar (Beta336/Beta4731) were sowed in each plastic tray with the above-mentioned growth condition in a 2cm deep furrow at 1cm apart. There were four replicates per growth condition and the experiment was set up as a completely randomized design. The greenhouse temperature during the experiment period was 27 ± 2 °C, with 80% relative humidity, and a 12-h photoperiod. Adequate water (approximately 500 ml) was ensured in every alternative day after sowing the seeds.
Figure 1: Two types of sugar beet seed were used for assessing germination
and phytotoxicity. A) Fungicide coated seed; B) Fungicide uncoated seed.
Statistical analysis
Statistical analysis consisted of one-way ANOVA with multiple comparisons testing performed by GraphPad PRISM 9.3.1. Experiments were done in four replicates and the data were plotted as the mean ± SE determinations.
Results
Fungicide-coated seeds and no holes underneath plastic trays showed the lowest seed germination at 27 days after sowing.
In vivo germination assessment of sugar beet seed “Beta 336X” under four growth conditions demonstrated significant variation. No fungicide coat on sugar beet seed, having holes underneath plastic trays showed 94% germination which was considered as check or control. No fungicide coat on seed, without having any hole’s underneath trays showed 70% germination. Fungicide coated seed, having holes underneath plastic trays showed 90% germination while fungicide coated seed, having no hole’s underneath trays- showed the lowest germination (≥20%) (Figure 2A).
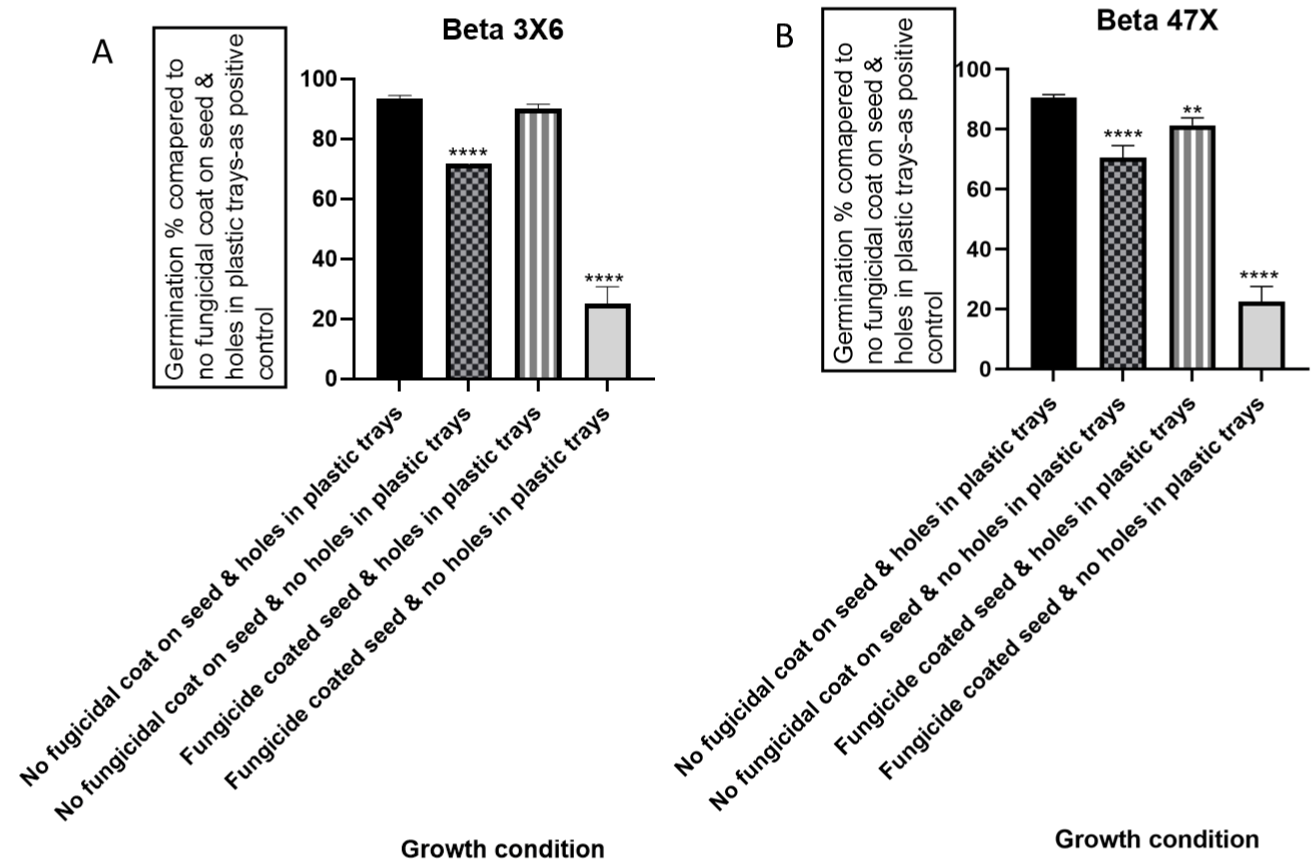
Figure 2: Germination percentage (%) at different growth conditions
compared to a positive control (no fungicidal coat on seed & holes in plastic
trays) with the no-fungicidal coat on seed & no holes in plastic trays, fungicidal
coated seed & holes in plastic trays, and fungicidal coated seed & no holes
in plastic trays. Germination % of (A) Beta 3X6; (B) Beta 47X. ****; ***; **; *
indicates significant differences in germination % compared to the control-
“no fungicidal coat on seed & holes in plastic trays” at p-value of ≤ 0.0001; ≤
0.001; ≤ 0.01; ≤ 0.05 respectively.
Likewise, sugar beet cv. “Beta 4731X” showed significant variation in germination percentage to different growth conditions. No fungicide coat on sugar beet seed, and hole underneath trays shows 90% germination. No fungicide coat on seed and no hole underneath trays showed 70% germination. Fungicide-coated seed and hole underneath trays showed 80% germination whereas fungicide-coated seed and no hole underneath trays- showed 20% germination (Figure 2B).
Fungicide-coated seeds, having no holes underneath plastic trays showed the highest percentage of stunted seedlings at 27 days after sowing
In vivo estimation of stunted seedlings (%) of sugar beet cv. “Beta 336X” and “Beta 4731X” under four growth conditions demonstrated significant variation. No fungicide coat on sugar beet seed and hole underneath plastic trays showed less than 2% stunted seedlings which were considered a positive control. No fungicide coat on seed and no hole underneath trays showed less than 5% stunted seedlings. Fungicide coated seed, and hole underneath plastic trays showed above 5% stunted seedlings while fungicide coated seed and no hole underneath trays-showed the highest percentage of stunted seedlings (<10%) in both cultivars (Figure 3A and 3B).
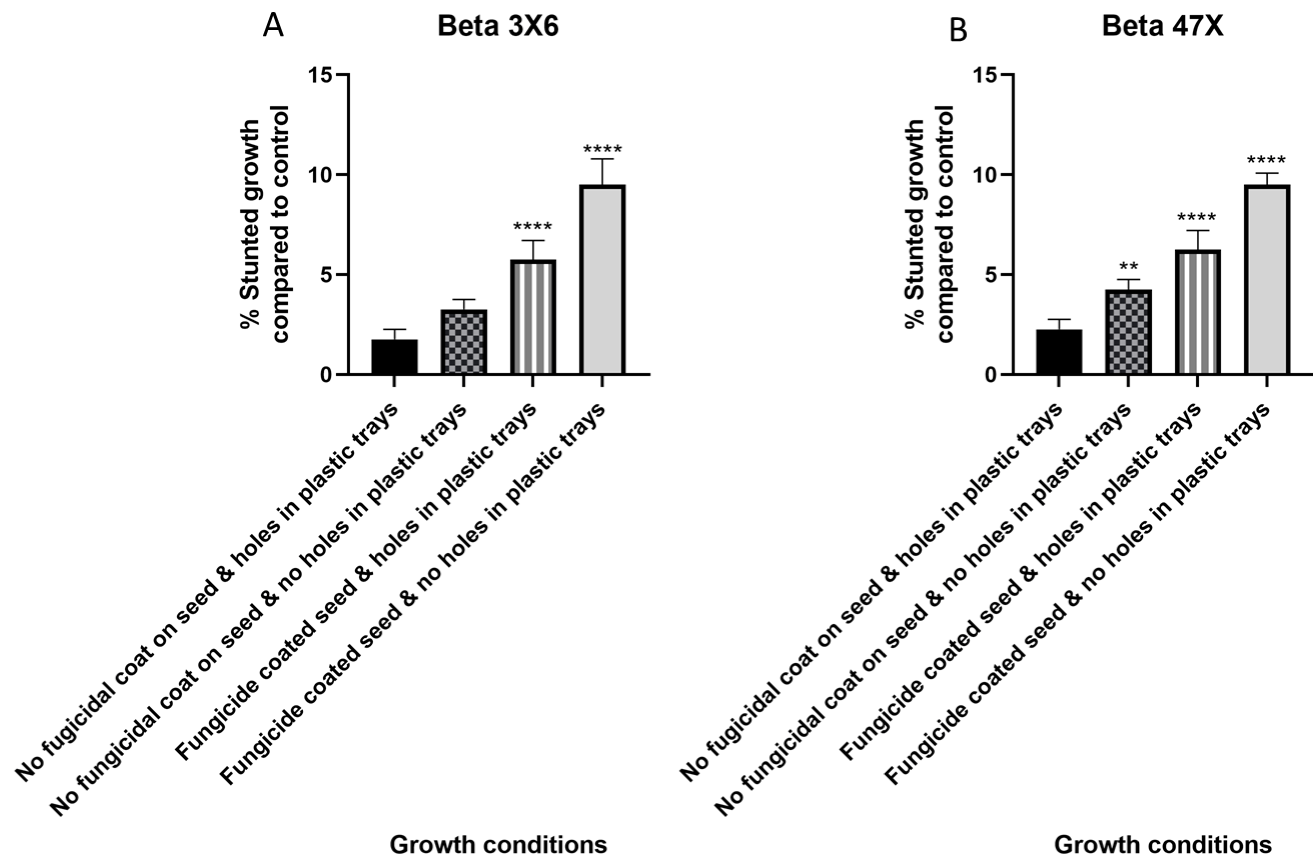
Figure 3: Percentage (%) of stunted seedlings at four growth conditions
compared to a positive control (no fungicidal coat on seed & holes in plastic
trays) with the no-fungicidal coat on seed & no holes in plastic trays, fungicidal
coated seed & holes in plastic trays, and fungicidal coated seed & no holes in
plastic trays. Percentage (%) of stunted seedlings of (A) Beta 3X6; (B) Beta
47X. ****; ***; **; * indicates significant differences in stunted seedlings (%)
compared to the “control no fungicidal coat on seed & holes in plastic trays”
at p-value of ≤ 0.0001; ≤ 0.001; ≤ 0.01; ≤ 0.05 respectively.
Discussion
Phytotoxicity results in poor germination, stunting, and delayed plant development. In this study, fungicide-coated seed, having no holes underneath the plastic tray showed the lowest seed germination in both beta seeds. The possible reason is likely to be phytotoxicity, this resulted in stunted growth and yellowing of seedlings. Other in vitro phytotoxicity study on Cattleya aurantiaca and Stanhopea occulata demonstrated the effect of fungicides, bactericides and dual-action substances on seedling growth and development [13]. Other research demonstrated that difenoconazole exposure caused oxidative stress, reduced chlorophyll biosynthesis and functions, and then inhibited wheat plant growth and development [9]. Another research found that seeds treated with silver nanoparticles showed (Ag-NPs) better germination but the seedling growth of tested specie was affected by exposure to concentrations of nanoparticles. Ag-NPs demonstrated significant potential in inhibiting soil borne fungi and bacteria presents on seeds and acts as an alternative source of fertilizer that may improve sustainable agriculture [14]. The highest germination was observed in uncoated fungicide seed and the holes underneath plastic trays which showed above 90% germination. This indicates no phytotoxic effect since the fungicide coat was removed prior to sowing; this resulted in normal plant growth. The holes in plastic trays help to remove excess water.
The moderate germination was observed in uncoated fungicide seed with no holes underneath plastic trays which showed above 70% germination. This provided evidence similar to soil hardpan or clay pan where surplus water cannot draining away freely and restricts root growth. Such a layer may be caused by ploughing at the same depth every year. Research has shown that hardpan effects the vertical distribution of water stress in a converted paddy field. Abiotic stress often intensifies with alternate prolonged wet and dry conditions in the surface and sub-surface layer above the hardpan, dry condition persisted after the hardpan dried [15]. Our research found relatively poor seed germination and sprouting because excess water could not leak out from the trays and resulting death of seedlings.
As expected fungicide-coated seed with holes underneath plastic trays showed 90% germination. Even though seeds were coated with fungicide, there was minimum phytotoxicity but it did not affect seed germination. Notably, delayed germination and a few abnormal growths were observed in each replicate. There was minimal abiotic stress because excess water leached out.
We also observed a marked increase of stunted seedlings in fungicide-coated seed with no hole underneath plastic trays. Lowest number of stunted seedlings shown in uncoated seed with holes. This further provided evidence of phytotoxicity which likely to be no hole’s in plastic trays. Other research demonstrated that Triazole-coated maize seed results in stunted seedlings under chilling. Additional findings shown that mesocotyl length reduced by 32.19–44.73% by difenoconazole, and 23.53-32.08% by tebuconazolet at rates of 1:50 and 1:25, respectively. The phytotoxicity of difenoconazole and tebuconazole is due to phytohormone imbalance [10]. Therefore, the seed companies need to consider evaluation of cultivars under various growth conditions to measure the phytotoxicity of fungicide coated seed.
Conclusion
Fungicides are an inevitable tool for soil borne sugar beet disease management. Hence, the development of new fungicides with minimum negative impact in sugar beet physiology is a future challenge.
Acknowledgments
Authors are very much thankful to BETASEED and M. F. R. Khan, Department of Plant Pathology, North Dakota State University, Fargo, ND 58102, University of Minnesota, St. Paul, MN USA for necessary suggestions and technical support.
References
- Gullino ML, Leroux P, Smith CM. Uses and challenges of novel compounds for plant disease control. Crop Protection. 2000; 19: 1-11.
- Saladin G, Magné C, Clément C. Effects of fludioxonil and pyrimethanil, two fungicides used againstbotrytis cinerea, on carbohydrate physiology invitis viniferal: Fungicides and carbohydrate physiology in grapevine. Pest management science. 2003; 59: 1083-1092.
- Belpoggi F, Soffritti M, Guarino M, Lambertini L, Cevolani D, Maltoni C. Results of long-term experimental studies on the carcinogenicity of ethylenebis- dithiocarbamate (mancozeb) in rats. Annals of the New York Academy of Sciences. 2002; 982: 123-136.
- Mendes CAC, Mendes GE, Cipullo JP, Burdmann EA. Acute intoxication due to ingestion of vegetables contaminated with aldicarb. Clinical toxicology (Philadelphia, Pa). 2005; 43: 117-118.
- Carter GA, Wain RL. Investigations on fungicides: Ix. The fungitoxicity, phytotoxicity, and systemic fungicidal activity of some inorganic salts. Annals of applied biology. 1964; 53: 291-309.
- Dias MC. Phytotoxicity: An overview of the physiological responses of plants exposed to fungicides. Journal of botany. 2012; 2012: 1-4.
- Kuretani M, Ueki K. Symptoms of phytotoxicity caused by application of dpa, ata and sodium chlorate on orchard crops. Zasso kenkyu. 1964: 72-76.
- Thomé A, de Souza TO, Thomé GCH, Reginatto C. Phytotoxic effect on corn and soybean due addition of nanoiron to the soil. Water, air, and soil pollution. 2000; 231.
- Liu R, Li J, Zhang L, Feng T, Zhang Z, Zhang B. Fungicide difenoconazole induced biochemical and developmental toxicity in wheat (Triticum aestivum l.). Plants (Basel). 2021; 10: 2304.
- Zhang C, Wang Q, Zhang B, Zhang F, Liu P, Zhou S, et al. Hormonal and enzymatic responses of maize seedlings to chilling stress as affected by triazoles seed treatments. Plant physiology and biochemistry. 2020; 148: 220-227
- Petit A-N, Fontaine F, Vatsa P, Clément C, Vaillant-Gaveau N. Fungicide impacts on photosynthesis in crop plants. Photosynthesis research. 2012; 111: 315-326.
- Sugar beet Rhizoctonia Management Plan for 2018.
- Thurston KC, Spencer SJ, Arditti J. Phytotoxicity of fungicides and bactericides in orchid culture media [cattleya aurantiaca and stanhopea occulata]. American journal of botany. 1979; 66: 825-835.
- Parveen A, Rao S. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. Journal of cluster science. 2014; 26: 693- 701.
- Hamada K, Inoue H, Mochizuki H, Miyamoto T, Asakura M, Shimizu Y. Effect of hardpan on the vertical distribution of water stress in a converted paddy field. Soil & tillage research. 2021; 214: 105161.