
Research Article
A Proteomics. 2015; 2(1): 1008.
Affinity of the Major Cationic Peanut Peroxidase Glycans to Lectins
Alsubaie M* and Huystee BV
Department of Biology, University of Western Ontario, Canada
*Corresponding author: Maha Alsubaie, Department of Biology, University of Western Ontario, Canada
Received: June 25, 2015; Accepted: September 24, 2015; Published: October 09, 2015
Abstract
The major Cationic Peanut Peroxidase (CPRx) contains heme, calcium and glycans the location of each of these moieties on the protein has been established following protein diffraction. This peroxides has 3 hybrid glycans chains, each with 5 different sugars and a total of 16. By using ESI-MS (electron spray ionization mass spec), it was noted that a wide variety of different glycan length forms were possible on the CPRx. However no further information on the prevalence of each form was found. When the space between the CPRx and glycosidases secreted by the cultured cells is relatively small, a rapid loss of sugars over limited time (i.e. 1 hour) is possible. In addition, where in the ESI-MS analysis the farthest sugars away from the N-185 protein binding site, were called hexoses, now it is clear that they are galactoses. The high amount of these relatively untouched glycans in the cell incubation medium, is probably due to the massive secretion of peroxidase by the large number of cells at the end of the 14 day culture period in a 1,5 L normal incubation medium. This examination of these sugar chains as well as the identity of the farthest sugars from the protein was made possible by lectin affinity.
Introduction
The major Cationic Peanut Peroxidase (CPRx) was found by 2-D electrophoresis to be one of only 27 detectible peptides released by cultured peanut cells over a 14 day culture period [1]. This small number of different proteins released, diminished the protein purification process for CPRx from the spent medium to isolation by Carboxy Methyl (CM) Sepharose chromatography [2]. With purification of this major isozyme achieved, as seen by 2D electrophoresis [3], the 3-D structure was obtained of the CPRx by X-ray diffraction of the crystal with its heme and calcium sites clearly visible [4]. That was a further support for the establishment of such structures of peroxidases from other plant species [5]. However, only the binding sites for three glycans on CPRx could be detected by X-ray diffraction [4]. The rest of the glycan chain probably tended to wave over the protein as shown in the case of RNase [6] The location of the three binding sites on CPRx had already been established by examining the glycopeptides from trypsin digested CPRx after obtaining its cDNA sequence [7]. Three glycopeptides following treatment of CPRx with try sin were isolated by gel filtration and their position on the protein was established at asparagines 60, -144, and -185 [8]. Then, with the help of HPLC on the isolated glycan chain it was also noted that 5 different sugars, namely N-acetyl-glucosamine, mannose, galactose, xylose and fucose are present in N-144 linkaged glycan [9]. By applying 1H-NMR, it was further confirmed that this is a hybrid glycan chain at N-144 with a total of 12 sugars incorporated [10]. At that time very little was still known about the fate of the glycans of peroxidase from other plant species, even a few years ago [11]. Yet, continued effort with ESI-MS has shown that all three glycans on the CPRx had similar chains with potential 16 sugars. However, they may be with variable sugar chains [12]. The variability in length can be attributed to the occurrence of glycosidases secreted by the peanut cells as was seen in the case of the assays for β-galactosidase [8]. But further verification is required for such assumption! Also that such loss of the glycans from the chains could be enhanced by decreasing the volume of the re-suspended spent medium pellet, without cells, drastically and the absence of cells for further releasing CPRx with complete glycans! That was the aim of this study. It also remains a project for CPRx to determine the role of the glycans [13,14].
Material and Methods
Protein isolation and identification
Peanut cells derived from sectioned seeds were cultured for 14 days in a culture medium [15] containing the required elements [16]. The spent suspension medium of 1,5 L, at the outset, after 14 days of cell culture, was next isolated by filtration and preliminary isolation of proteins was achieved by addition of acetone to 70% saturation. Then, the CPRx was isolated by chromatography on a CM Sepharose column with a 0.075 M acetate buffer pH 5 [2]. This peroxidase isozyme was identified from the column eluate by its reaction in 0.1 ml, with two drops of 0.3% H2O2 and two drops of 1.0% guaiacol. Its reaction was measured at 470 nm, as well as its high absorption at 280 and 403 nm for protein and heme in each fraction [2].
The loss of sugars in a confined volume
The crude precipitate of the 1,5 liter spent medium was initiated in 70% acetone, as the first step for the further isolation steps of CPRx mentioned above. However, now the non-fractioned pellet was re-suspended in only 3 ml 0.05 M sodium phosphate buffer pH 5 and kept for increasing time periods at room temperature at a 500 greater concentration than normal. At each determined time, being 5 minutes, 1 ml 98% H2SO4 and 0.2 ml 5% phenol was added to 20 μl of sample to remove the proteins and then to determine sugars in the remaining solution by addition of sulphuric acid and 5% phenol [17]. Protein amounts to be used were determined from a specific quantity, obtained from a graph at 280 nm produced by increasing amounts of commercial Horse Radish peroxidase
In the next part of the study the CPRx protein obtained by the CM Sepharose was passed through a Sephadex G-75 filtration before lectin affinity.
Lectin affinity
Four types of lectin columns (2.5 x 0.5 cm) were employed for the determination of exposure of sugars on the glycan chains [18]. In all cases a 2ml amount of lectin was used as prescribed by EY Lab Inc for their lectin kit. The column was equilibrated in a 10 ml TCM (0.01 M Tris- HCl buffer at pH 7.5 containing 0.5M NaCl and 1mM CaCl2, MgCl2 and MnCl2) [8]. The protein for the Con-A and RCA lectins, at 0.35 ng in 2 ul, to be examined was taken from the first 3 fractions of the Sephadex G-75 column as mentioned above in gel filtration. The loaded column was then washed with 10 ml of TCM saline buffer while absorbance at 280 nm was measured. Next, for either the Con-A column or the Ricinus Communis (RCA) the potential fractions were eluted with 0.2 M mannose or 0.2 M galactose as suggested by EY laboratories Inc. In the case of the Triticum Vulgare (WGA) lectin affinity the elution buffer was 0.1M N-acetyl glucosamine, and for the Ulex Europaeus (UEA-1) was 0.05M a-fucose all in a TCM saline buffer pH 7.5. For the latter two analyses the protein sample was 0.16 ng protein from the original Sephadex G-75 column.
Results
The purification of CPRx by CM Sepharose column chromatography was identical to that shown before [2], the elution of the gel filtration column of the original fraction showed large amounts of high molecular weight peptides [19] with a high sugar content at the first effluent of this G-75 column. However, when the non-fractionated 14 day old spent medium, acetone precipitate, was incubated over time in a 3 ml sodium phosphate pH 5 solution, it is seen that a major sugar amount was released already in 1 hour. No further increase of sugars was noted after this one hour incubation period this liberated sugar presence started at extreme low at the beginning of the test and rose rapidly with time to a maximum within one hour. That may be seen as a rapid action of the glycosidases also present in the 14 day spent medium, as earlier β-galactosidase was detected [8]. It also explains why there may be such great variety in chain lengths from 16 to 4 sugars, as seen by ESI-MS [12].
Then, when lectin affinity was applied to the purified CMSepharose CPRx, there was for the Con-A column some attached protein as seen after the addition of mannose. (Figure 1). This observation is a confirmation of earlier data with this approach [8]. However, this is relatively low when compared to the affinity of CPRx to the RCA column, (Figure 2), indicating high quantities of exposed galactose compared to mannose to be present in the intact glycan chains of CPRx. That is even more noted for the comparisons of the WGA and UAE columns (Figure 3 and 4) which have only a very minor peak. The conclusion of such is that the galactose is a major exposed sugar for the secreted CPRx with some for mannose but barely any for the N-acetylglucoseamine and fucose. Yet, minor exposure is seen as was also noted with the 1H-NMR and ESI-MS date on side branches for the glycans [10,12].
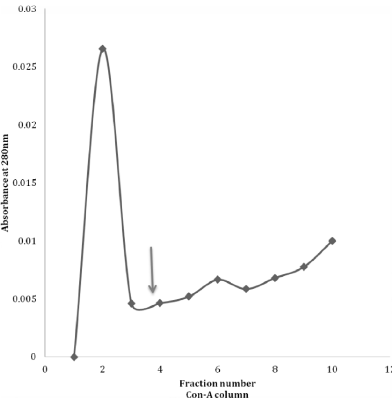
Figure 1: Lectin (Concanavaline-A) Con-A column affinity of CPRx with
protein detection at 280nm. At the arrow 5ml of 0.2 mannose was added.
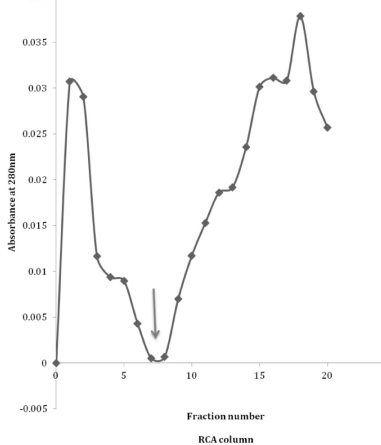
Figure 2: Lectin (Ricinis communis) RCA column profile of CPRx with 5ml 0.2
M mannose added at the arrow.

Figure 3: Lectin (Wheat germ agglutinin) WGA column of CPRx with 5ml
0.1M N-acetylglucoseamine added at the arrow.

Figure 4: Lectin (Ulex europaeus) UEA-1 column of CPRx with 5 ml 0.05M
fucose added t the arrow.
Discussion
This study is largely based on the initial sugar data of the middle glycan chain N-144 composition [9], as well as the relation of these sugars to each other in the hybrid chain [10] and thus the heterogeneity of these chains [12]. In addition, it also solves the question as to the type of sugars at the end of the three glycan chains that have been identified only as hexoses [12]. The glycan chains detected by ESI-MS were larger than the initial chain proposed [9]. That may be due to the cleaving action of β-glycosidase [8] and others. The high affinity of CPRx to the RCA lectin in this study suggests that there is a large amount of galactose exposed. That also agrees with the high effect of β-galactosidase on CPRx in lowering its MW [8] and the major loss of 3 kDa of the three glycan chains of the two species from 40 to 37 kDa [19]. In that reaction, the fraction 40 kDa called then CP- and the other 37 kDa fractions CP+ were based on their affinity to the Con-A column being positive for the 37 kDa fraction with exposed mannose. However, the CP+ fraction was only 10 % of the CP- fraction i.e. 90 % was the protein itself and the remaining glycan! The potential question why the glycosidases do not work more effectively can be explained by the relatively large 1.5 liter volume of the incubation medium [15] and the rapid growth of the cells in the medium over 14 days. The high and rapid release of sugars from CPRx, when incubated in a small volume without cells, confirms the large amount of glycans on the protein suggested to be 20% of MW of CPRx [19] and the prevalence of other glycosidases than galactosidase. Yet the slight affinity of the CPRx to the other two lectin columns also indicates that, xylose and N- acetyl glucosamine may have limited exposure in the intact glycan chains [12] but their exposed presence in the chain is minimal compared to galactose. But it can be brought about with exposure to glycosidases as observed by the high amount of sugars liberated in one hour in a small volume of assay medium. A variety of sugar analysis techniques for the determination on glycosidic chains of proteins was already suggested earlier [6]. It has also been reported that in peanut nodules there are mannose and galactose binding lectins [20].
In general, those lectins may be used as a means to determine the exposed sugars of hybrid glycoprotein, has a wide influence in medical research [21] even in cancer studies [22]. It now is also of interest to determine n how far the protein structure is altered by the loss of sugars, since the loss of calcium already causes some alterations, particular around the enzyme active site [23]. The loss of a single glycan by site-directed mutagenesis showed already some loss of enzyme activity as well as thermal stability [24]. An overall view of the role of this peroxidase with its components has been presented before [25].
Acknowledgement
We want to thank Drs Gbric V, Trick C and Gijzen M. For their help. Similarly we want to also express our appreciation to Chung and Y, Dr Timoshenko A.
References
- van Huystee RB, Tam ASK. Peptides released by cultured peanut cells during growh J. Plant Physiol. 1988; 33: 645-647
- Maldonado BA, van Huystee RB. Isolation of a cationic peroxidase from cultured peanut cells. Can. Bot. 1980; 58: 2280-2284.
- Hu C, Krol M, van Huystee RB. Comparison of anionic with Cationic peroxidase from cultured peanut cells. Plant Cell, Tissue and Organ Culture. 1990; 22: 65-70.
- Schuller DJ, Ban N, Huystee RB, McPherson A, Poulos TL. The crystal structure of peanut peroxidase. Structure. 1996; 4: 311-321.
- Dunford HB. Heme Peroxidases. John Wiley and Sons. Inc-VCH. 1999.
- Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996; 240: 210-226.
- Buffard D, Breda C, van Huystee RB, Asemota O, Pierre M, Ha DB, et al. Molecular cloning of complementary DNAs encoding two cationic peroxidases from cultivated peanut cells. Proc Natl Acad Sci U S A. 1990; 87: 8874-8878.
- Wan L, Gijzen M, Van Huystee RB. Heterogeneous glycosylation of cationic peanut peroxidase. Biochem Cell Biol. 1994; 72: 411-417.
- Sun Y, Lige B, van Huystee RB. HPLC determination of the sugar composition of the glycans on the cationic peanut peroxidase J. Agric Food Chem. 1997; 45: 4196-4200.
- Shaw GS, Sun Y, Barber KR, van Huystee RB. Sequence specific analysis of the heterogeneous glycan chain from peanut peroxidase by 1H-NMR spectroscopy. Phytochemistry. 2000; 53: 135-144.
- Dunford HB. Peroxidases and Catalases. John Wiley and Sons. Inc-VCH. 2O10.
- Zhang C, Doherty-Kirby A, Huystee Rv Rv, Lajoie G. Investigation of cationic peanut peroxidase glycans by electrospray ionization mass spectrometry. Phytochemistry. 2004; 65: 1575-1588.
- van Huystee RB, Sun Y, Lige B. Glycans of peroxidase, do they have a special role? Current topics in Biochem. Res. 1999; 1: 165-171.
- van Huystee RB, Roig MG, Shnyrov VL, Sakharov IY. Peroxidase stability related to its calcium and glycans. Phytochemistry Rev. 2004; 3: 19-28.
- Verma DPS, van Huystee, RB. Cellular differentiation and peroxidase isozymes in cell culture of peanut cotyledons. Can.J. Bot. 1970; 48: 429-431.
- Linsmaier JF, Skoog F. Organic growth factor requirements of tobacco tissue culture. Physiol. Plant. 1965; 18: 100-127
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 1956; 28: 350-356.
- Sharon N, Lis H. Lectins 2nd Ed. Kluwer Acad. Publ. 2003.
- O'Donnell JP, Wan L, van Huystee RB. Characterization of two forms of cationic peroxidase from cultured peanut cells. Biochem Cell Biol. 1992; 70: 166-169.
- Law IJ, van Tonder HJ. Localization of mannose and galactose-binding lectins in and effective peanut nodule. Protoplasma. 1992; 167: 10-18.
- Montreuil J, Vliegenthart JFG, Schachter H. Glycoproteins and disease. New comprehensive biochem. 1996.
- Gabius HJ, Nagel GA. Lectins and glycol-conjugates in oncology. Springer-Verlag. 1988.
- Maranon MJR, van Huystee RB. Plant peroxidases: Interaction between their prosthetic groups. Phytochemistry. 1994; 37: 1217-1225.
- Lige B, Ma S, van Huystee RB. The effects of the site-directed removal of N-glycosylation from cationic peanut peroxidase on its function. Arch Biochem Biophys. 2001; 386: 17-24.
- van Huystee RB, Sun Y, Lige B. A retrospective look at the cationic peanut peroxidase structure. Crit Rev Biotechnol. 2002; 22: 335-354.