
Research Article
Austin J Public Health Epidemiol. 2023; 10(1): 1139.
Paricalcitol Ameliorated Dextran-Sulfate-Sodium-Induced Colitis in Mice through MKP-1/P38 MAPK Signaling Pathway
Liyuan Lv1#, Guinan Liu1#, Man Yang1#, Tao Chen2, Xiang Li2, Jun Zhang2, Jie Liu2, Yi Liu2*
¹Department of Gastroenterology, The Seventh Affiliated Hospital of Sun Yat-sen University, Guangdong, China.
²Department of Gastroenterology, Huashan Hospital, Fudan University, Shanghai,China.
#Equal Contribution.
*Corresponding author: Yi LiuHuashan Hospital, Fudan University, Shanghai, China
Received: November 28, 2022; Accepted: January 09, 2023; Published: January 16, 2023
Abstract
Background and Aim: Ulcerative Colitis (UC), a type of inflammatory bowel disease, of which the accurate pathogenesis is not yet well understand. Recently, the Vitamin D/VDR signaling pathway and the activated vitamin D analogues have been proved as playing important role in the pathogenesis of UC. In the present study, our objective was to evaluate the effect of Vitamin D analogues paricalcitol on dextran-sulfate-sodium-induced colitis in a mouse model.
Methods: We evaluated the effects of the activated vitamin D analogues paricalcitol on the development of Dextran-Sulfate-Sodium-(DSS)-induced colitis. Clinical symptoms were evaluated by the Disease Activity Index (DAI) and tissue samples were evaluated by Histopathological Scoring (HS). Meanwhile, the mucosal mRNA expression of cytokines, Tumor Necrosis Factoralpha (TNF-a), Interleukin-6 (IL-6), Interleukin-10 (IL-10), Interleukin-17 (IL-17) were analyzed by real-time semi quantitative reverse transcription polymerase chain reaction. The mucosal protein VDR, p38 Mitogen-Activated Protein Kinase (P38-MAPK) and Mitogen-Activated Protein Kinase Phosphatase-1 (MKP-1) expressions of the vitamin D/VDR signaling pathway were analyzed using Western blot.
Results: The results showed that the weight loss and colon length shortening of DSS-induced mice were significantly improved after paricalcitol treatment. In addition, both DAI and HS were significantly reduced. Paricalcitol down regulated the mucosal expression of messenger RNA of pro-inflammatory cytokines TNF-a, IL-6 and IL-10 and upregulated anti-inflammatory cytokines IL-17. Both VDR protein expression and MKP-1 level increased, whereas the mucosal expression of p38-MAPK was found to be decreased.
Conclusion: Activated Vitamin D analogues paricalcitol can ameliorate the development of DSS-induced colitis through the Vitamin D/VDR signaling pathway.
Keywords: Ulcerative colitis; Paricalcitol; Vitamin D Receptor (VDR); P38 MAPK; P-P38 MAPK.
Introduction
Inflammatory Bowel Disease (IBD) is associated with chronic relapsing inflammation of the intestinal tract of unknown etiology. UC and Crohn’s Disease (CD) are the two major forms of IBD. Recent studies have indicated that a complex interplay of genetic, microbial, and environmental factors culminates in sustained aberrant intestinal innate immunity, which may be a central early mechanism, subsequently perpetuated by dysregulated adaptive immune responses [1].
Apart from its classical calcium-regulating effect, vitamin D serves as a potent regulator of multiple biological activities, including antimicrobial activities, the inhibition of apoptosis and immunomodulatory functions [2]. We both know that the majority of the body’s vitamin D content is derived from pho tosynthesis in the skin following UV light irradiation.1,25-Dihydroxyvitamin D3 (1,25(OH)2D3) is the active form of vitamin D and binds with VDR. Recent studies have shown that VDR is highly expressed in Intestinal Epithelial Cells (IECs) [3], indicating that the vitamin D/VDR signaling pathway may play a key role in the process of IBD. Increasing data have suggested VDR gene polymorphisms are associated with the incidence of IBD [4,5] and VDR knockout mice have been shown to have a compromised mucosal barrier, leading to increased susceptibility to mucosal damage and an increased risk of developing IBD [6].
The vitamin D/VDR signaling pathway, commonly referred to as the P38MAPK pathway, involves a series of proteins and gytokines. The dual-specificity MKPs comprise a subfamily of 10 catalytically active proteins, including MKP-1, has been shown to regulate p38 via dephosphorylation to restrain over-activation of MAPK and to keep homeostasis. Thus, in this study, to clarify the role of the vitamin D/VDR signaling pathway in intestinal inflammation, we evaluated the effects of activated vitamin D analogues paricalcitol on the development of DSS-induced colitis. Our data demonstrated that the P38-MAPK, TNF-a, IL-6 and IL-17 expression in the mice with DSS-induced colitis were down regulated by the treatment of paricalcitol. However, the expression of IL-10, VDR and MKP-1 were upregulate.
Materials and Methods
Animals and Groups
Thirty male BALB/c mice (SPF level) purchased from an experimental animal center (Shanghai Medical College, Fudan University, China), all weighing about 25g,were randomly assigned into five groups: the normal control group (Group A ), the UC model group (group B), the low-dose (0.1ug/kg body weight) intervention group (group C), the moderate-dose (1ug/kg body weight) intervention group (group D) and the large-dose (10ug/kg body weight) intervention group (group E). All animal procedures were reviewed and approved by the Laboratory Animal Ethics Committee of Fudan University.
Main Reagents
Dilute DSS (from MP Biomedicals: M.W=36,000-50,000,60316ES25) with distilled water into 5% DSS solution.Paricalcitol Injection (from Abbott, CAS No.: 131918-61-1). RNA extraction kit (from TRIzol Reagent. Life Technologies, USA). Real-time quantitative polymerase chain reaction (Realtime qPCR) Kit (from TOYOBO ReverTra Ace® qPCR RT Kit Code No. FSQ-101, Japan). Reverse transcription polymerase chain reaction kit (from TOYOBO SYBR Green Real-time PCR Master Mix Code No.QPK-201, Japan). Anti-p38MAPK (9F12; sc-81621, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Anti-P-P38MAPK (sc-101758; Santa Cruz Biotechnology Inc. Santa Cruz, CA, USA). Anti-MKP-1 (sc-370, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
Modeling of Ulcerative Colitis
All mice were fed in a standard laboratory for one week before modeling. In accordance with the classic method used by Cooper [7] Group B to Group E were given 5.0% (w/v) DSS solution as drinking water for days 1-7 to induce acute intestinal inflammation while the normal control group drank distilled water freely. Group C, D and E received paricalcitol by intragastric administration once on days 1, 4 and 7 with 0.1ug/kg paricalcitol each time for the group C, 1ug/kg paricalcitol each time for the group D,and with 10ug/kg paricalcitol each time for the group E. Group A and group B received ddH2O intragastric administration as control. All mice were killed on the eighth day and four colon specimens from each mouse were dissected from the different sections of colon for further detection (one for nucleic acid, one for cytokines, one for proteins, one for histopathology).
Evaluation of Symptoms and Physical Signs
The daily Disease Activity Index (DAI) assessment was carried out according to the classic scoring system by Cooper [7] in the process of modeling. DAI is the sum of weight loss, trait of stool, and occult blood in stool or hematochezia.
Evaluation of Histopathology Inflammation
The whole colons were harvested on day 8. The distal colon were taken and fixed in 10% formaldehyde solution overnight at 4OC, dehydrated with graded alcohol and then embedded in paraffin for histological analysis. Sections of the colon (5 um) were stained with hematoxylin and eosin. Microscopic scoring was independently by two pathologists who were blinded to the group design and the classic scoring system by Cooper [7] was carried out. The colonic damage was categorized into six segments: severity and extent of inflammation, degree of regeneration, degree of crypt damage and percentage involvement. Total scores are the sum of the scores of each individual segment.
RNA Isolation and Real-time qPCR
Total RNA was isolated by the guanidinium isothiocyanate method followed by centrifugaion. Total RNA concentration was quantified by ultraviolet spectrophotometer three times. A260nm/A280nm was 1.8-2.0.RNA was conserved at -80OC in the fridge, β-actin was selected to span an intron. Quantities of mRNA for β-actin, TNF-a, IL-6, IL-10, IL-17, VDR, p38 MAPK and MKP-1 were determined by Real-time qPCR using the sequences of the primers designed by Primer Express (Table 1). Initial denaturation at 95OC for 3min, followed by 40cycles consisting of denaturation at 95OC for 5s, primers annealing at 60OC for 30sOC and elongation at 72OC for 45s. The Fluorescent quantitation was completed by Funglyn FTC-3000 Real-time PCR System. The result of each experimental group was repeated for three times.
Primer name
Forward(5'→3')
Reverse(3'→5')
TNF-a
ACCACGCTCTTCTGTCTACTGA
GGTTTGTGAGTGTGAGGGTCTG
IL-6
TTCCATCCAGTTGCCTTCTTGG
ACAGGTCTGTTGGGAGTGGTAT
IL-10
GCTCTTACTGACTGGCATGAGG
TTCCGATAAGGCTTGGCAACC
IL-17a
CTGTGTCTCTGATGCTGTTGCT
TGGAACGGTTGAGGTAGTCTGA
VDR
GGCTTCCACTTCAACGCTATGA
GCCTCTTCCTCCTTCCTCTTCA
p38 MAPK
CAGTGCCTATCACGCTTCTCG
GTCTGCCTTGTGGTTGTCCTC
MKP-1
CGAGAGTTGCGTCTGCTGAA
CTGGCACTTCACGATGTTGTTC
β-actin
GCTACAGCTTCACCACCACAG
GAACCGCTCGTTGCCAATAGT
TNF: Tumor Necrosis Factor; IL: Interleukin; VDR: Vitamin D Receptor; p38 MAPK: p38 Mitogen-Activated Protein Kinase; MKP-1: Mitogen-activated Protein Kinase Phosphatase-1
Table 1: Primers used in this study for PCR.
Determination of P38-MAPK, P-P38MAPK and MKP-1: Western blot analysis
Total protein concentration is quantified by ultraviolet spectrophotometer. An equal amount of total protein of 30ul per lane was fractionated on a 10% Sodium Dodecyl Sulfate (SDS)-polyacrylamide gel. Gels were transferred to polyvinylidene difluoride membranes after electrophoresis. The membranes were blocked with Tris-buffered saline containing 5% nonfat dry milk at 4OC, then incubated overnight at 4OC in 0.1% Tween 20 with 5% nonfat dry milk with antiP38-MAPK,antiP-P38MAPK and anti-MKP-1 at 1:200, 1:500, 1:200 dilutions. Following washing in TBS-T for three times, membrances were visualized using the TANON GIS-2008 formatter and analysis was came out using the GIS Image Processing System. Samples were assessed for Glyceraldehyde-3-Phosphate Dehydrogease (GAPDH) content as an internal control. The ratios of integrate of target proteins and GAPDH were determined as the correspondent expression of the target proteins. The result of each experimental group was repeated for three times.
Statistical Analysis
Data is shown by x±s, and statistical analysis was carried out using SPSS 18.0.Significance was considered at a P<0.05 level.
Results
DAI Evaluation
The DAI scores were the lowest in Group A, which is the normal control group. DAI scores were relatively higher than Group A in Groups B-E that exposure to DSS induced diarrhea, weight loss, rectal bleeding and sparse hair. Group B, the UC model group was the most different form the normal control group (A versus B, P<0.05). Both Group C and Group D had relatively lower score than Group B, but there were no significant differences between each group (C/D versus B, P>0.05). Most notably, Group E, the large-dose intervention group, showed a markedly decreased score than Group B (E versus B, P<0.05) (Figure 1).
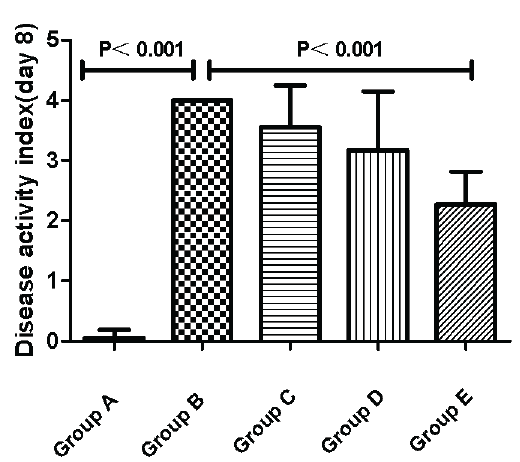
Figure 1: Effects of activated Vitamin D analogues paricalcitol on disease activity index at day 8.Data are represented by mean±standard error of the mean. Normal control group (A), Ulcerative Colitis (UC) model group (B), the low-dose intervention group (C), the moderate-dose intervention group (D) and the large-dose intervention group (group E).
Histopathological Score Evaluation
The Histopathological Score was zero in Group A. Compared to Group A, Group B showed a significant difference. The inflammation was improved from Group C to Group E which was the paricalcitol intervention groups. There were no significant differences between Group C and Group B (C versus B, P>0.05). However, both Group D and Group E were markedly different to Group B (D/E versus B, P<0.05) (Figure 2).
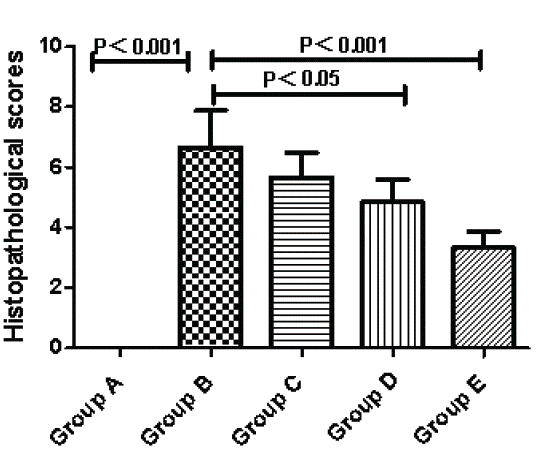
Figure 2: Effects of activated Vitamin D analogues paricalcitol on histological scoring at day 8. Normal control group (A), Ulcerative Colitis (UC) model group (B), the low-dose intervention group (C), the moderate-dose intervention group (D) and the large-dose intervention group (E).
The mRNA Expressions of TNF-a, IL-6, IL-10, IL-17, VDR, P38 MAPK and MKP-1
The mRNA expression of pro-inflammatory cytokines TNF-a, IL-6 and IL-17 were markedly increased in Group B, but in Group D and Group E the levels became conspicuously lower (D/E versus B, P<0.05). Anti-inflammatory cytokines IL-10 in Group C, Group D and Group E had relatively higher mRNA expression than Group B, but there were no statistically significant differences between each group. We found that in mice with DSS-induced colitis, expressions of VDR were much lower than normal mice while intervention with paricalcitol could upregulate VDR expressions in Group C-E(C/D/E versus B, P<0.05).The mRNA expressions of P38 MAPK were the highest in Group B and showed a significant difference compared with intervention groups(C/D/E versus B, P<0.05). There were no significant differences with the mRNA expressions of MKP-1 between Group A and Group B, however, Group B showed a markedly lower mRNA expressions of MKP-1 than intervention groups (C/D/E versus B,P<0.05) (Figure 3).
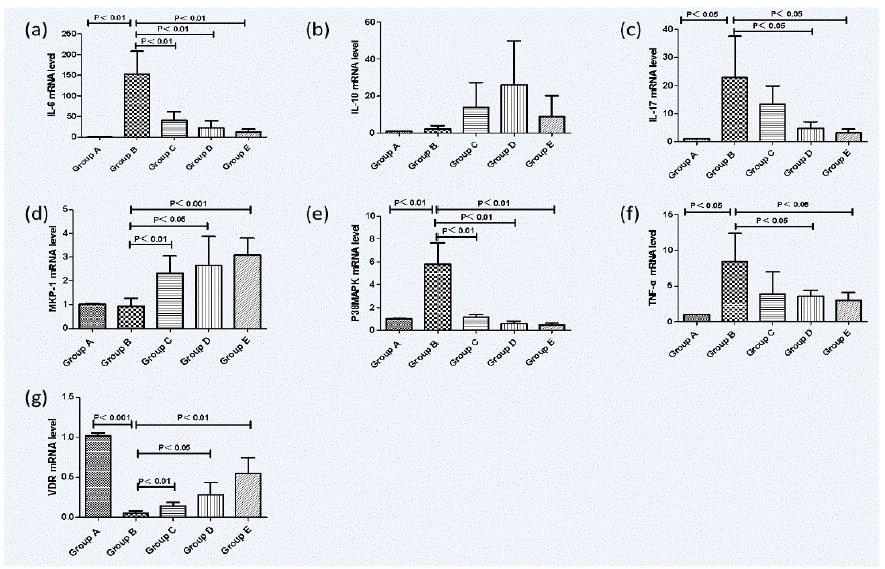
Figure 3: Real-time polymerase chain reaction (PCR) analysis of mucosal cytokine mRNA expression in dextran-sulfate-sodium-induced colitis. Normal control group (A), Ulcerative Colitis (UC) model group (B), the low-dose intervention group (C), the moderate-dose intervention group (D) and the large-dose intervention group (E). (a) The mRNA expression of pro-inflammatory cytokines IL-6. (b) The mRNA expression of anti-inflammatory cytokines IL-10. (c) The mRNA expression of pro-inflammatory cytokines IL-17. (d) The mRNA expression of MKP-1. (e) The mRNA expression of P38MAPK (f) the mRNA expression of pro-inflammatory cytokines TNF-a. (g) The mRNA expression of VDR.
The Protein Expressions of PP38 MAPK, P38 MAPK, and MKP-1
The expressions of PP38 MAPK in Group B were significantly higher than Group A (P<0.01). However, Intervention with paricalcitol could reduce their levels in a dose-dependent manner, as indicated by Groups C, D and E. The expression levels of P38 MAPK in Group B, Group C, Group D and Group E were nearly the same as that in Group A and there was no statistical significance between each group (P>0.05). The protein expressions of MKP-1 similar to the result of mRNA expressions of MKP-1 what we had already showed. (D/E versus B, P<0.05) (Figure 4).
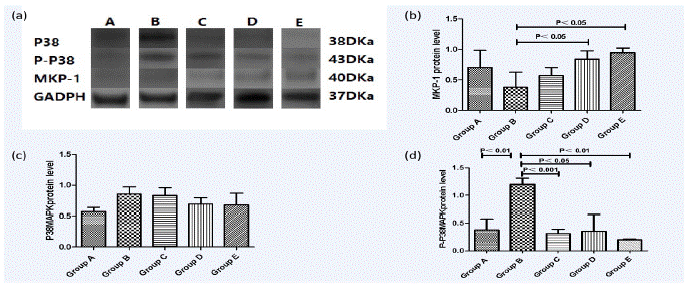
Figure 4: Western-blot analysis of P38MAPK, P-P38MAPK and MKP-1 expression in dextran-sulfate-sodium-induced colitis. P38MAPK, P-P38MAPK and MKP-1 were measured using ultraviolet spectrophotometer. Data are represented by mean±standard error of the mean (n=6 mice/group). Normal control group (A), Ulcerative Colitis (UC) model group (B), the low-dose intervention group (C), the moderate-dose intervention group (D) and the large-dose intervention group (E). (a) Western-blot for expressions of P38MAPK,P-P38MAPK and MKP-1 in representative mice treated with DSS and/or paricalcitol. (b) Changes of MKP-1 expression. (c) Changes of P38MAPK expression. (d) Changes of P-P38MAPK expression.
Discussion
Accumulating evidence indicates an important role of vitamin D not just critical to mineral and skeletal homeostasis but in reducing the risk of developing several chronic inflammatory or autoimmune conditions, such as multiple sclerosis, type 1 diabetes and rheumatoid arthritis [8-10]. After vitamin D being metabolized to its active form, 1, 25 (OH) 2D3, exerts its genomic actions by binding and activating a nuclear transcription factor, VDR [11]. VDR is highly expressed in the intestine, Kong et al [12] first reported that vitamin D deficiency compromises the mucosal barrier, leading to increased susceptibility to mucosal damage and an increased risk of developing IBD. Moreover, epidemiological evidence suggests a close relationship with between vitamin D deficiency and an increased risk of developing IBD [13-15]. Results showed that paricalcitol, an activated vitamin D analogue, could alleviate the severity of DSS-induced colitis in mice, as shown by significant improvement in body weight loss, inflammatory infiltration, colon length shortening and tissue damage.
Various intracellular proteins can initiate inflammation.p38 proteins are a class of mitogen-activated protein kinases (MAPKs) that are major players during inflammatory responses [16]. Lipopolysaccharide (LPS), a component of the Gram-negative bacterial cell wall, induces cytokine production by monocytes/macrophages. The MAPKs activated by LPS (ERK, JNK and P38 [17]) are critical regulators of pro-inflammatory cytokine production, including TNF-a and IL-6, IL-10 [18,19]. It is generally accepted that inflammatory cytokines play an important role in the pathogenesis of IBD. In healthy individuals, these cytokines act as guards against any invading pathogens [20]. MKP-1 is known to preferentially inactivate P38MAPK, leading to subsequent inhibition of proinflammatory cytokines production [21,22] Zhang Y et al [23] found that vitamin D inhibits LPS-induced cytokine production by up-regulating MKP-1 thereby attenuating p38MAPK activation. On the other hand, P38MAPK is a key linkage in the VDR signaling way. Our laboratory data provide a therapeutic foundation for enhancing vitamin D/VDR signaling to inhibit intestine al inflammation. The expressions of TNF-a and IL-6 and IL-10 were down regulated after the treatment with activated vitamin D analogues paricalcitol on DSS-induced colitis. Our data showed that the expression of substance P38MAPK is abnormal in DSS-induced colitis, and were significantly down-regulated after being treated with paricalcitol. We also found that in mice with DSS-induced colitis, expressions of VDR were much lower than normal mice while intervention with paricalcitol could upregulate VDR expressions. Therefore, this indicated that the vitamin D/VDR signaling pathway might be activated in treatment of paricalcitol, expressions of MKP-1 were upregulated which significantly suppressed the activation of P38MAPK,in this way, it attenuated the release of pro-inflammatory cytokines and relieved inflammatory responses in the colon to prevent the development of DSS-induced colitis.
Taken together, such preliminary data confirm that activated vitamin D analogues paricalcitol can prevent the development of DSS-induced colitis and may become a potential candidate in such therapeutic regimes.
Acknowledgments
I would like to express my gratitude to my supervisor Prof. Yi Liu for his great support on my project. Thanks to his guidance and help, I was able to complete my entire work. I also want to thank the research team for their collaboration and help during gathering data for my research project.
Declaration of Interest Statement
All authors have no conflicts of interest.
References
- Zhang SZ, Zhao XH, Zhang DC. Cellular and molecular immunopathogenesis of ulcerative colitis. Cell Mol Immunol. 2006; 3: 35-40.
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008; 29: 726-776.
- Tong Zhu, Tian-Jing Liu, Qun Zhao, Shi Yong-Shi. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Molecular Medicine. 2015; 35: 1213-1218.
- Wang L, Wang ZT, Hu JJ, Fan R, Zhou J, Zhong J. Polymorphisms of the vitamin D receptor gene and the risk of inflammatory bowel disease: a meta-analysis. Genet Mol Res. 2014; 13: 2598-2610.
- Xue LN, Xu KQ, Zhang W, Wang Q, Wu J, Wang XY. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: a meta-analysis. Inflamm Bowel Dis. 2013; 19: 54-60.
- Kong J, Zhang ZY, Musch MW, Ning G, Sun J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008; 294: 208-216.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69: 238-49.
- Cutolo M, Paolino S, Sulli A, Smith V, Pizzorni C, Seriolo B. Vitamin D, steroid hormones, and autoimmunity. Ann NY Acad Sci. 2014; 1317: 39-46.
- Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med. 2014; 239: 1524-1530.
- Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004; 229: 1136-1142.
- Brumbaugh PF, Haussler MR. Specific binding of 1alpha,25-dihydroxycholecalciferol to nuclear components of chick intestine. J Biol Chem. 1975; 250: 1588-94.
- Kong J, Zhang ZY, Musch MW, Ning G, Sun J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008; 294: 208-216.
- Tan B, Li P, Lv H, Li Y, Wang O, Xing Ping X, et al. Vitamin D Levels and bone metabolism in Chinese adult patients with inflammatory bowel disease. J Dig Dis. 2014; 15: 116-123.
- Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011; 56: 830-836.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004; 126: 1504-1517.
- Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, et al. Functional Roles of p38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediators of Inflammation. 2014; 352371: 1155-68.
- Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000; 12: 20–26.
- Kracht M, Saklatvala J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 2002; 20: 91–106.
- Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008; 63: 784-790.
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001; 13: 85-94.
- Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006; 203: 1883-1889.
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-in lipopolysaccharide-stimulated macrophages. J Immunol. 2002; 169: 6408-6416.
- Zhang Y, Leung DY, Richer BN, Liu Y, Remigio LK, et al. Vitamin D Inhibits Monocyte/macrophage Pro-inflammatory Cytokine Production by Targeting Mitogen-Activated Protein Kinase Phosphatase 1. J Immunol. 2012; 188: 2127-2135.