
Research Article
Austin J Public Health Epidemiol. 2023; 10(3): 1150.
An Epidemiological Study of Aeromonas spp. Infections in Norway
Rohringer A1,4; Syre H2; Hyllestad S3; Amato E3*
1Department of infection control and vaccine, Norwegian Institute of Public Health, Norway
2Department of Medical Microbiology, Stavanger University Hospital, Norway
3Department of infection control and preparedness, Norwegian Institute of Public Health, Norway
4European Programme for Public Health Microbiology Training (EUPHEM), European Centre for Disease Prevention and Control (ECDC), Stockholm, Sweden
*Corresponding author: Amato E Department of Infection Control and Preparedness, Norwegian Institute of Public Health, 0456, Oslo, Norway. Email: Ettore.Amato@fhi.no
Received: June 12, 2023 Accepted: July 12, 2023 Published: July 19, 2023
Abstract
Aeromonas spp. is ubiquitous in aquatic habitats causing a wide range of infections in humans after exposure to contaminated water or food. We conducted an epidemiological study of Aeromonas infections detected in Norway, using laboratory-based surveillance data during 2014-2018, in order to identify risk factors associated with developing a severe infection. We identified 503 Aeromonas cases over a 5-year period with an average incidence of 1.9 per 100,000 inhabitants per year. Aeromonas mostly caused gastrointestinal infections (69.8%, n=351), followed by wound (8.6%, n=43) and blood infections (7.4%, n=37). Gastrointestinal and wound infections peaked in the summer months. Major isolated species were A. hydrophila (15.3%), A. veronii (10.7 %), and A. caviae (10.7%). Hospitalisation was reported for 81.1% of blood infections (n=30), 51.2% of wound infections (n=22) and 23.1% of gastrointestinal infections (n=81). Risk factors for gastrointestinal infections associated with hospitalisation were (i) age group 65-79 years old (adjOR=3.10; 95% CI: 1.39-6.93) and >80 years old (adjOR=17.66; 95% CI: 5.05-61.79) and (ii) infections caused by A. caviae (adjOR=3.26; 95% CI: 1.3-8.1). This study showed that Aeromonas infections are common throughout the years suggesting a diverse and continuous source of exposure. Future research on environmental sources and preventive measures particularly for severe Aeromonas infections is recommended.
Keywords: Aeromonas spp.; Epidemiology; Risk factors; Waterborne infections; Norway
Introduction
The genus Aeromonas belongs to the Aeromonadaceae family and comprises a group of Gram-negative bacteria widely distributed in aquatic environments, with some species able to cause disease in humans, fish, and other aquatic and terrestrial animals [1-3]. Among the 36 species described in the genus Aeromonas, four species (Aeromonas caviae, Aeromonas dhakensis, Aeromonas veronii and Aeromonas hydrophila) have more frequently been reported in clinical cases than other Aeromonas spp. [4].
Aeromonas spp. are considered opportunistic pathogens that can cause a wide range of mild to severe infections, including gastrointestinal (GI), wound and blood infections, in both immunocompromised and immunocompetent hosts [5-7]. The source of infection is usually contaminated food or water [8,9]. Particularly, Aeromonas spp. have been isolated from a variety of food sources including meat [10,11], milk [12,13], vegetables [11], shellfish [11,14] and fish [3,11,13,15]. Moreover, it can be present in drinking water systems, where Aeromonas spp. readily form biofilms with other heterotrophic bacteria tolerating chlorination and even pipe cleaning [16,17].
The epidemiology of Aeromonas infections in not well studied, cases are sporadic rather than associated with large outbreaks and data are therefore derived from a limited number of studies [18]. Only a few studies reporting the incidence on the national level have been published. In those studies, the overall reported or estimated incidence range from 0.2 in France to 9.9 cases per 100,000 inhabitants in Australia [5,19,20].
The aim of this study was to describe the epidemiology of Aeromonas cases to determine the burden of these infections in Norway and identify risk factors associated with developing severe infection for this opportunistic waterborne pathogen.
Materials and Methods
Study Design and Case Definition
We conducted a retrospective study to analyse the epidemiology of Aeromonas spp. infections in Norway during the period 2014-2018. A case of Aeromonas infection was defined as a laboratory confirmed Aeromonas spp. from a clinical sample.
Data Source and Collection
We collected data on Aeromonas infections through a nation-wide survey sent to Norwegian medical microbiology laboratories. Twenty-one laboratories representing the whole country were invited to participate in this study, of which 16 participated by submitting the number of cases and related epidemiological information. The following variables were collected: patients’ sex, age group, year and month of infection, region of residence, identified Aeromonas species, type of sample, infection type, underlying conditions, coinfections, and hospitalisation status. Species identification was performed through Matrix Assisted Laser Desorption Ionization-Time of Flight mass spectrometry (MALDI-TOF) following their routine clinical procedures.
Epidemiological Investigation
We described the epidemiology of Aeromonas infections by sex, age group, geographic distribution by county and region, time of infection, identified species, seasonality, and severity of infection. Severity of infection was inferred from data on hospital admission.
Aeromonas infections were classified into 8 groups based on their site of isolation: (i) blood infections including blood samples; (ii) GI infections including faecal samples; (iii) wound infections including purulent exudate in surgical wound and wound swabs, (iv) ear infections including ear swab samples, (v) skin infections including skin swabs, (vi) urinary tract infections including urine samples and urinary tract/bladder biopsies, (vii) pulmonary infection including sputum, tracheobronchial aspirate, or lung biopsy (pneumonia), (viii) other infections including cholangitis, peritoneal exudate or intraabdominal abscess pus (peritonitis) and unknown infections and sample types.
Seasons were defined according to the northern hemisphere seasons (spring: March – May; summer: June – August; autumn: September – November; winter: December – February). Population data per year were publicly available from national statistics [21].
Data and Statistical Analysis
For each independent variable (sex, age group, season, region, and species), we estimated crude Odds Ratios (OR) and 95% CI of hospitalisation by univariate logistic regression analysis. Adjusted ORs (adjOR) with 95% CI were estimated in a Multivariate Analysis (MVA). Binary outcome was hospitalised/non hospitalised Aeromonas infection. Observations with missing values for variables under comparison were excluded from the respective analysis. We used an alpha level of 0.05 for all statistical tests (Stata version 16.0, 2019. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP. USA). STATA outputs of p-values p<0.000 are reported as p<0.001.
Ethical Considerations
Ethics committee approvals was obtained in line with internal procedures and the General Data Protection Regulation (ethical approval 2019/123/REK sør-øst C on 13.03.19 and its revision on 21.11.2019 and 01.03.2023). Only aggregated data were analysed in this study.
Results
Epidemiology of Aeromonas Infections in Norway
Sixteen out of the 21 invited medical microbiology laboratories replied to the national survey on total number of Aeromonas cases during the 5-years study period, reaching a response rate of 76% and 503 unique Aeromonas cases.
Our data showed that Aeromonas infections were reported with an average of 101 cases per year (range: 88-117) and an overall incidence of 1.9 per 100,000 inhabitants per year. The number of cases followed a seasonal distribution, peaking each year during the summer months (n=178, 35.4%) (Figure 1). Aeromonas infections were more frequently reported from females (n=266, 52.9, %, male to female ratio 0.9), and in the age group 45-64 (n=140, 27.8%). While the highest incidence was reported in the elderly more than 65 years old, and in children below 4 years old (Figure 2). The most common type of infection reported was GI infection (n=351, 69.8%) followed by wound (n=43, 8.6%) and blood (n=37, 7.4%) (Table 1).
Gastrointestinal infections (n=351)
Wound infections (n=43)
Blood infections (n=37)
Other infections1 (n=72)
Total (n=503)
n
%
n
%
n
%
N
%
Total
Sex
Male
160
45.6
24
55.8
16
43.2
32
44.4
232
Female
191
54.4
19
44.2
16
43.2
40
55.6
266
Unknown
0
0
0
0
5
13.5
0
0
5
Age group
(0-4)
40
11.4
0
0
0
0
2
2.8
42
(5-14)
27
7.7
0
0
0
0
8
11.1
35
(15-24)
24
6.8
8
18.6
0
0
3
4.2
35
(25-44)
92
26.2
10
23.3
1
2.7
11
15.3
114
(45-64)
101
28.8
12
27.9
8
21.6
19
26.4
140
(65-79)
48
13.7
10
23.3
10
27.0
22
30.6
90
(80+)
19
5.4
3
7.0
13
35.1
7
9.7
42
Unknown
0
0
0
0
5
13.5
0
0
5
Season
Spring
67
19.1
5
11.6
3
8.1
13
18.1
88
Summer
119
33.9
21
48.8
11
29.7
27
37.5
178
Autumn
84
23.9
14
32.6
7
18.9
17
23.6
122
Winter
64
18.2
3
7.0
11
29.7
15
20.8
93
Unknown
17
4.8
0
0
5
13.5
0
0
22
Species
A. caviae
26
7.4
2
4.7
16
43.2
10
13.9
54
A. hydrophila
42
12.0
18
41.9
2
5.4
15
20.8
77
A. veronii
40
11.4
2
4.7
6
16.2
6
8.3
54
Aeromonas spp.
238
67.8
21
48.8
12
32.4
35
48.6
306
Other species
5
1.4
0
0
1
2.7
6
8.3
12
Coinfection
No
169
48.1
14
32.6
6
16.2
13
18.1
202
Yes
36
10.3
5
11.6
8
21.6
17
23.6
66
Unknown
146
41.6
24
55.8
23
62.2
42
58.3
235
Underlying condition
No
127
36.2
18
41.9
11
29.7
17
23.6
173
Yes
24
6.8
8
18.6
8
21.6
16
22.2
56
Unknown
200
57.0
17
39.5
18
48.6
39
54.2
274
Hospitalisation
No
257
73.2
18
41.9
2
5.4
29
40.3
306
Yes
81
23.08
22
51.2
30
81.1
32
44.4
165
Unknown
13
3.7
3
6.9
5
13.5
11
15.28
32
Region2
North
19
5.6
1
2.6
3
9.4
5
7.5
28
Centre
55
16.2
5
12.8
2
6.3
5
7.5
67
West
204
60.2
17
43.6
14
43.8
29
43.3
264
South
36
10.6
7
18.0
3
9.4
10
14.9
56
East
25
7.4
9
23.1
10
31.3
18
26.9
62
Unknown
12
3.5
4
10.3
5
15.6
5
7.5
26
Note 1: other infections include cholangitis, peritonitis, and unknown infections or sample types due to their frequency of less than 20 cases during the 5-year period.
Note 2: Geographic region includes the following counties: Troms og Finnmark and Nordland (North); Trøndelag and Møre og Romsdal (Centre); Vestland and Rogaland (West); Adger (South); Innlandet, Oslo, Viken and Vestfold og Telemark (East)
Table 1: Summary of epidemiological parameters of Aeromonas cases per type of infection, Norway, 2014-2018.
Characteristics
Hospitalised cases
Non-hospitalised cases
Univariate Analysis
Multivariate Analysis
All cases (N=338)
n
%
n
%
OR (95% CI)
adjOR (95% CI)
81
24
257
76
Sex
Female
40
11.83
144
42.6
0.78 (0.49-1.26)
0.87 (0.51-1.47)
Male
41
12.13
113
33.43
1
-
Age group
0-4
6
1.78
33
9.76
0.87 (0.33-2.29)
0.86 (0.32-2.29)
14-May
6
1.78
17
5.03
2.42 (0.95-6.16)
2.03 (0.75-5.47)
15-24
7
2.07
16
4.73
2.06 (0.76-5.55)
2.03 (0.73-5.66)
25-44
17
5.03
74
21.89
1
-
45-64
13
3.85
85
25.15
0.77 (0.37-1.62)
0.85 (0.39-1.83)
65-79
17
5.03
28
8.28
2.94 (1.36-6.35)
3.10 (1.39-6.93)
80+
15
4.44
4
1.18
15.42 (4.56-52.08)
17.66 (5.05-61.79)
Season1
Spring
13
3.85
50
14.79
0.75 (0.35-1.60)
0.67 (0.29-1.59)
Summer
31
9.17
83
24.56
0.95 (0.49-1.84)
0.99 (0.48-1.59)
Autumn
14
4.14
67
19.82
0.56 (0.26-1.18)
0.57 (0.25-1.32)
Winter
19
5.62
44
13.02
1
-
Species
A. hydrophila
8
2.37
29
8.58
1.42 (0.34-2.92)
1.51 (0.69-3.27)
A. caviae
11
3.25
13
3.85
3.17 (1.39-7.24)
3.26 (1.32-8.05)
A. veronii
6
1.78
30
8.88
1.05 (0.49-2.29)
0.85 (0.34-2.12)
Aeromonas spp.
56
16.57
181
53.55
1
-
Other species
0
0
4
1.18
1
-
Note 1: Data on the variable season was available only for 321 cases.
Table 2: Univariate and multivariate analysis of predictors for developing severe gastrointestinal Aeromonas infections.

Figure 1: Average number of Aeromonas infections per season and infection type, Norway, 2014-2018.
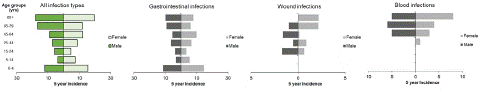
Figure 2: Incidence of Aeromonas infections by age-group and sex, Norway, 2014-2018. 5-year incidence of (A) all infection types (B) gastrointestinal infections (C) wound infections (D) blood infections.
Gastrointestinal Infections
GI cases were the most frequently reported Aeromonas infection during the 5-years study period, with an average number of 70 cases and an incidence of 1.4 per 100,000 inhabitants reported per year. GI infections were more frequently reported in females (n=191, 54.4%), with a male to female ratio of 0.8 and the highest number of cases in adults 45-64 years old (n=101, 28.8%). However, the highest incidence was reported in children below 4 years old (13.1 per 100,000) and in elderly more than 80 years old (8.6 per 100,000). Twenty-four cases reported underlying conditions, mostly cancer, gastric bypass surgery and colitis. Infections followed a seasonal distribution peaking during the summer months (n=119, 33.9%). Aeromonas spp. were detected from faecal samples and not subtyped at species level for most of the cases (n=238, 67.8%). When subtyped, the most reported species was A. hydrophila (n=42, 12.0%), followed by A. veronii (n=40, 11.4%). Thirty-six cases reported coinfections mostly associated with Campylobacter, followed by Salmonella, Shigella, Streptococcus, Klebsiella and Clostridium spp. Almost all cases (96.3%) reported information on hospitalisation and about a quarter (24.0%) were hospitalised. The western region reported the highest number of cases (n=204, 60.2%) followed by the centre, southern, eastern, and northern regions (Table 1).
Wound Infections
Wound infections were the second most reported Aeromonas’s type of infection during the 5-years study period with an average number of 8.6 cases and an incidence of 0.2 per 100,000 inhabitants reported per year. Wound infections were more frequently reported in males (n=24, 55.8%), with a male to female ratio of 1.3, and in all age groups except infants and children below 15 years old. The highest number of cases was reported for the age group 45-64 (n=12, 27.9%), however the highest incidence was reported in the 56–79-year-olds (1.6 per 100,000). Eight cases reported underlying conditions such as complications from wound healing, gangrene, chronic wounds and amputations. Infections followed a seasonal distribution with a peak of cases during summer months (n=21, 48.8%). Aeromonas spp. were detected from open wounds, biopsies and exudate samples, and not subtyped at species level for a large number of cases (n=21, 48.8%). When subtyped, the most reported species was A. hydrophila (n=18, 41.9%) and in lower percentage A. veronii and A. caviae (for both n=2, 4.7%). Five cases reported coinfections mostly associated with Proteus and Staphylococcus spp. More than half of the cases (55%) reported information on hospitalisation and 7.5% were hospitalised. The western region reported the highest number of cases (n=17, 43.6%) followed by the eastern, southern, centre and northern regions (Table 1).
Blood Infections
Blood infections were the third most reported type of Aeromonas infection during the 5-years study period with an average number of 7.4 cases and an incidence of 0.1 per 100,000 inhabitants reported per year. Blood infections were equally distributed between males and females. No blood infections were reported in the age groups below the age of 25, while an increasing number of cases was reported by age-group with the highest number of cases in elderly above 80 years old (n=13; 35.1%). Eight cases reported underlying conditions such as cancer and gallbladder infections. Infections did not follow a seasonal pattern, with a low number of cases reported throughout the seasons. Aeromonas spp. were detected from blood samples and subtyped at species level for many cases (n=12, 32.4%) reporting A. caviae as the predominant detected species (n=16, 43.2%). Eight cases reported coinfections mostly associated with Klebsiella spp. and Escherichia coli.
Almost all cases (93.1%) reported information on hospitalisation and 81.1% were hospitalised. The western region reported the highest number of cases (n=14, 43.8%) followed by the eastern, southern, northern, and centre regions (Table 1).
Other Infections
Other Aeromonas infections included ear infections (n=14, 2.8%), urinary tract infections (n=11, 2.2%), respiratory infections (n=9, 1.8%), skin infections (n=8, 1.6%), and other infections such as appendicitis, cholecystitis, eye infections and peritonitis (n=30, 6%). Epidemiological parameters are available in Table 1.
Risk Factors for Developing Severe Aeromonas Gastrointestinal Infections
Our unmatched multi variate analysis comparing the non-hospitalised with the hospitalised cases of Aeromonas GI infections revealed that sex and seasons do not play a significant role in the risk of developing severe infection. While we observed that the proportion of severe cases increased significantly with increasing age, particularly for elderly in the age group 65-79 years old (OR=3.1; 95% CI: 1.39-6.93) and more than 80 years old (OR=17.7, 95% CI: 5.05-61.79), as well as with A. caviae as GI-causing species (OR=3.3, 95% CI: 1.32-8.05) (Table 3).
Discussion
This epidemiological study provides an overview of the occurrence of Aeromonas infections in Norway between 2014-2018. The overall incidence of Aeromonas infections was calculated to 1.9 per 100,000 inhabitants per year. Incidence of Aeromonas infections from different countries is scarcely reported and vary from 0.2 to 9.9 per 100,000 in France and Australia, respectively [4,20]. A similar incidence to Norway, 1.1 per 100,000, was reported from a study in California, USA. [7]. While many factors influence the overall incidence of Aeromonas in any given country, higher incidence may be linked to warmer and tropical climates which present favourable growth conditions for Aeromonas (20-37°C) [22].
The main infection types of Aeromonas observed in Norway were GI, wound and blood infections. GI infections were also reported among the majority of Aeromonas cases in the Californian study [7], however both the French [5] and Australian studies [20] reported a majority of wound infections.
The incidence of Aeromonas causing GI infections in Norway was 1.1 per 100,000 inhabitants per year. The highest incidence rate was observed in children 0-4 years and elderly above 80 years, highlighting that these age groups are more vulnerable for developing GI infections. The reported GI infections followed a seasonal pattern peaking in the summer with an average of 24 cases per year. The annual cases of GI infections, over the study period stayed relatively stable with an average of 70 cases per year (range 54-78), this points towards a continuous exposure. Aeromonas GI infections are associated with the consumption of contaminated food and water. Observations may be a result of the combination of increased Aeromonas concentration and likelihood for exposure during the summer months [20]. Most cases (60%) in Norway have been reported from the Western region with an incidence of 3.1 per 100,000 per year. To contextualise this value, we can compare with other gastrointestinal infections that are mandatorily notifiable in Norway such as Campylobacter and Salmonella reporting an incidence of 73.8 and 21.2 cases per 100,000 per year from the same region over the same time period [23].
Aeromonas spp. infections are not mandatorily notifiable in Norway, however the higher number of cases reported in the Western region suggests that infections with this opportunistic pathogen are relatively common. Cases may also go undetected as Aeromonas spp. are not routinely tested for but rather are detected in the procedure to exclude more commonly tested pathogens. Therefore, the detection of Aeromonas could be influenced by laboratory sensitivity based on their testing methods, algorithms and procedures.
Regarding risk factors associated with GI infection requiring hospitalisation, our multivariate model showed that the odds of developing GI infections that require hospitalisation is 3.1 and 17.7 times higher in the age groups 65-79 and above 80 years old, respectively compared to other age groups. While patients suffering from GI infections caused by A. caviae were 3.3 times more likely to be hospitalised. These findings may be explained with the increased likelihood of underlying diseases with increasing age and point towards a higher pathogenicity of A. caviae compared to other species.
Wound infections were the second most reported Aeromonas-associated infections. These types of infections were more frequently reported in males of all age groups except in infants and children less than 15 years of age. Although the yearly average number of wound infections was relatively low, the reported cases peaked in 2018 with 18 cases. These cases were reported from all regions of Norway. However, most of those cases were reported in the summer months like GI infections, suggesting a common source of infection such as contaminated water. The trend of an increased number of cases reported during the warmer months was also observed in the Australian context [20]. This is also in line with the co-occurrence of an increase in other waterborne pathogens like Vibrio infections in 2018 reported for the Nordic countries and attributed to the exceptionally warm climate [24].
For blood infections, only few cases were reported, and no clear seasonality was observed however cases clustered in summer and winter with an average of 2 cases per year for each season. There were also no significant peaks of cases observed over the study period with an annual average of 7.4 cases (range 5-11). These findings may point towards different risk factors associated with blood infections compared to GI and wound infections in Norway and seem to be mostly correlated with increased age particularly for elderly above 80 years old. This is in line with previous observations highlighting the importance of age as a risk factor for this type of infection [5].
Severe infections often lead to a higher rate of hospitalisation, which in turn is a burden on the health care system. The percentage of hospitalisation with all types of Aeromonas infections over the study period averages around 32.7% (29.6-36.5) per year which shows that Aeromonas infections requires hospital care in almost a third of the reported cases. The number of hospitalised cases increased by age; this could be explained by underlying conditions also increasing by age group in our dataset.
As expected, the percentage of hospitalised cases was highest for Aeromonas causing blood infections (81.1%) followed by wound (51.0%) and GI infections (23.1%). Like the hospitalisation trend, Aeromonas spp. have been more frequently subtyped for blood infections (67.6%) followed by wound (51.2%) and GI infections (32.2%). These results are expected due to prioritization of subtyping for samples from severe cases.
Overall, the most frequently isolated species detected in patient samples were A. hydrophila (n=77, 15.3%), A. veronii (54, 10.7%) and A. caviae (54, 10.7%). However, due to limitations of the identification methods such as MALDI-TOF [25], the percentage of A. hydrophila could be overestimated. The number of reported cases attributed to these species varied between the years and no trend or pattern was identified. A. hydrophila was more frequently detected in GI (12%) and wound infections (41.9%) while A. caviae was the most frequently detected species in blood infection samples (43.2%). This finding, combined with the increased likelihood of hospitalisation in GI infections caused by this species, suggests that A. caviae is likely the most invasive Aeromonas spp. in Norway. Although the Australian and French study [5,20] showed similar results, other countries like Taiwan and Japan reported A. hydrophila as the most common species causing blood infections [26,27]. Coinfections were reported for 66 cases where Campylobacter spp. was the most common detected pathogen, however further research is needed to clarify the role of Aeromonas in coinfections.
The limitations of this study include sampling bias towards severely ill, older, or comorbid individuals. Consequently, the age distribution of cases could be biased for mild infections, and thus underreported. In case of hospital admission, the reasons for hospitalisation were also not reported. Furthermore, Aeromonas infections are not mandatorily notifiable in Norway, therefore the data in this study were provided by the laboratories based on a voluntary survey, resulting in a reporting bias and completeness limitations for some of the collected parameters such as coinfections and underlaying conditions. Also, Aeromonas is not routinely tested for at medical microbiological laboratories in Norway and is typically only identified after excluding more common pathogens. The observed increase in the Western region highlights an important aspect of the laboratory testing performed for Aeromonas. Our investigation revealed that one laboratory had a significantly higher number of Aeromonas cases compared to other laboratories, which may be a result of different internal laboratory procedures, like culture media and testing practices [19,28]. Results of our study rely on a high response rate from the laboratories, with 76% of laboratories providing information on number of cases and epidemiological variables. However, the number of reported cases largely varied between laboratories with a range of 3-202 cases reported per laboratory.
Conclusion
This study has shed light on the epidemiology of Aeromonas infections in Norway and given insight into possible risk factors associated with infections. The study has shown that GI infections are the predominant infection type in Norway. The highest cumulative incidence was reported in infants and the elderly, and the even distribution of cases in space, time and sex suggests a constant and prolonged environmental exposure. The risk of developing severe infections increased by causing species (A. caviae) and increasing age. Future research and source attribution study investigating potential environmental sources of Aeromonas infections, such as water for human consumption and use, would be valuable to understand the transmission’s dynamics.
Author Statements
Financial Support
The project has been financially supported by the European Programme for Public Health Microbiology Training (EUPHEM), ECDC. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The views and opinions presented in this article are those of the authors and do not represent the opinion of the ECDC.
Data Availability Statement
Only fully anonymized data (i.e., data that are neither directly nor potentially indirectly identifiable) are permitted to be shared publicly. Therefore, legal restrictions prevent the authors from publicly sharing the dataset used in the study.
Acknowledgements
We would like to acknowledge Loredana Ingrosso (EUPHEM ECDC Fellowship
Programme) and Umaer Naseer for revising this manuscript. We thank the Norwegian microbiology laboratories network for their kind collaboration in providing data.
Authors’ Contributions
AR performed the epidemiological analysis and prepared the first draft manuscript. HS provided data from Stavanger area. EA collected the data at national level and supervised the project. EA, SH and HS advised during epidemiological analysis and interpretation. All authors contributed to the manuscript, revised and approved the final version.
Data Availability Statement
Only fully anonymized data (i.e., data that are neither directly nor potentially indirectly identifiable) are permitted to be shared publicly. Therefore, legal restrictions prevent the authors from publicly sharing the dataset used in the study.
References
- Beaz-Hidalgo R, Figueras MJ. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J Fish Dis. 2013; 36: 371-88.
- Laviad-Shitrit S, Izhaki I, Arakawa E, Halpern M. Wild waterfowl as potential vectors of Vibrio cholerae and Aeromonas species. Trop Med Int Health. 2018; 23: 758-64.
- Gowda TK, Reddy VR, Devleesschauwer B, Zade NN, Chaudhari SP, et al. Isolation and seroprevalence of Aeromonas spp. Among common food animals slaughtered in Nagpur, Central India. Foodborne Pathog Dis. 2015; 12: 626-30.
- Fernández-Bravo A, Figueras MJ. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms. 2020; 8.
- Lamy B, Kodjo A, colBVH Study Group, Laurent F. Prospective nationwide study of Aeromonas infections in France. J Clin Microbiol. 2009; 47: 1234-7.
- Wu CJ, Chen PL, Tang HJ, Chen HM, Tseng FC, et al. Incidence of Aeromonas bacteremia in Southern Taiwan: vibrio and Salmonella bacteremia as comparators. J Microbiol Immunol Infect. 2014; 47: 145-8.
- King GE, Werner SB, Kizer KW. Epidemiology of Aeromonas infections in California. Clin Infect Dis. 1992; 15: 449-52.
- Gonçalves Pessoa RB, de Oliveira WF, Marques DSC, Dos Santos Correia MT, de Carvalho EVMM, et al. The genus Aeromonas: A general approach. Microb Pathog. 2019; 130: 81-94.
- Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. Sci World J. 2012; 13.
- Tsheten T, Tshering D, Gyem K, Dorji S, Wangchuk S, et al. An outbreak of Aeromonas hydrophila food poisoning in Deptsang Village, Samdrup Jongkhar, Bhutan, 2016. J Res Health Sci. 2016; 16: 224-7.
- Neyts K, Huys G, Uyttendaele M, Swings J, Debevere J. Incidence and identification of mesophilic Aeromonas spp. from retail foods. Lett Appl Microbiol. 2000; 31: 359-63.
- Yucel N, çitak S. The occurrence, hemolytic activity and antibiotic susceptibility of motile Aeromonas spp. Isolated from meat and milk samples in turkey. J Food Safety. 2003; 23: 189-200.
- Nishikawa Y, Kishi T. Isolation and characterization of motile Aeromonas from human, food and environmental specimens. Epidemiol Infect. 1988; 101: 213-23.
- Hoel S, Vadstein O, Jakobsen AN. The significance of mesophilic Aeromonas spp. in minimally processed ready-to-eat seafood. Microorganisms. 2019; 7: 91.
- Hoel S, Mehli L, Bruheim T, Vadstein O, Jakobsen AN. Assessment of microbiological quality of retail fresh sushi from selected sources in Norway. J Food Prot. 2015; 78: 977-82.
- Sen K, Rodgers M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: a PCR identification. J Appl Microbiol. 2004; 97: 1077-86.
- Gavriel AA, Landre JP, Lamb AJ. Incidence of mesophilic Aeromonas within a public drinking water supply in north-east Scotland. J Appl Microbiol. 1998; 84: 383-92.
- Salvat M, Ashbolt N. Aeromonas. 2019.
- Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010; 23: 35-73.
- McAuliffe GN, Hennessy J, Baird RW. Relative frequency, characteristics, and antimicrobial susceptibility patterns of Vibrio spp., Aeromonas spp., Chromobacterium violaceum, and Shewanella spp. in the Northern Territory of Australia, 2000-2013. Am J Trop Med Hyg. 2015; 92: 605-10.
- SSB. Population in Norway 4th quarter 2021. SSB. Vol. 2022. Contract No.: 05.12.2021.
- Rouf MA, Rigney MM. Growth temperatures and temperature characteristics of Aeromonas. Appl Microbiol. 1971; 22: 503-6.
- Folkehelseinstituttet. Norwegian Surveillance System for Communicable Diseases (MSIS). 2022. Contract No.: 23.03.2022.
- Amato E, Riess M, Thomas-Lopez D, Linkevicius M, Pitkänen T, et al. Epidemiological and microbiological investigation of a large increase in vibriosis, northern Europe, 2018. Euro Surveill. 2022; 27.
- Latif-Eugenín F, Beaz-Hidalgo R, Figueras MJ. Evaluation of different conditions and culture media for the recovery of Aeromonas spp. from water and shellfish samples. J Appl Microbiol. 2016; 121: 883-91.
- Hirai J, Hagihara M, Kinjo T, Haranaga S, Fujita J. Blood stream infections due to Aeromonas species: A retrospective analysis of 24 cases at a Tertiary Hospital in Okinawa And previous related literature review in Japan. Open Forum Infect Dis. 2016: 3.
- Tang HJ, Lai CC, Lin HL, Chao CM. Clinical manifestations of bacteremia caused by Aeromonas species in southern Taiwan. PLOS ONE. 2014; 9: e91642.
- Abbott SL, Cheung WKW, Janda JM. The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. J Clin Microbiol. 2003; 41: 2348-57.