
Research Article
Austin J Public Health Epidemiol. 2024; 11(1): 1158.
Peripheral Neuroblastic Tumors in A Series of 82 Children: First Epidemiological Report from Morocco
Tabyaoui I1; Serhier Z2; El Maani K3,4; Cherkaoui S5,6; Madani A6; Bennani-Othmani M5; Tahiri Jouti N1*
1Laboratory of Cellular and Molecular Inflammatory, Degenerative and Oncologic Pathophysiology, Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Morocco
2Laboratory of Clinical Neurosciences and Mental Health, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
3Laboratory of Clinical Immunology, Inflammation and Allergy, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
4Department of Pediatrics III, “Harouchi Children’s Hospital”, Casablanca, Morocco
5Laboratory of Cellular and Molecular Pathology, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
6Department of Hematology and Pediatric Oncology, 20 Août 1953 Hospital, Casablanca Morocco
*Corresponding author: Nadia Tahiri Jouti, PhD Laboratory of Cellular and Molecular Inflammatory, Degenerative and Oncologic Pathophysiology - Hassan II University of Casablanca - Faculty of Medicine and Pharmacy, 19 rue Tarik Ibnou Ziad – BP 9154 - Mers Sultan – Casablanca, Morocco. Tel: +00 212 6 66 02 75 59 Email: nadiatahiri19@gmail.com
Received: January 31, 2024 Accepted: March 10, 2024 Published: March 18, 2024
Abstract
Background: The aim of this study was to report some clinical and histopathological characteristics of Peripheral Neuroblastic Tumors (pNTs) in our country since no data on these intriguing neoplasms has been reported so far in Morocco.
Methods: Files of 82 children diagnosed at the three major public hospitals of Casablanca, Morocco between 2007 and 2010 were reviewed for clinical, pathological and epidemiological characteristics.
Results: The annual incidence was 14.2 per million children with a median age at diagnosis of 38.6 months. The M/F sex ratio was 1.5. Only 31.7 % of cases presented at less than 18 months of age and 29.2% cases had low stage disease (I, II, and IV-S). In 39% of cases, tumors were extended beyond the primary site. Based on the International Neuroblastoma Pathology Classification (INPC), 58.5% of pNTs showed unfavorable histology. 71% of patients whose follow-up data were available died. Among them, 3 of 4 had relapsed. The overall survival at 3 years was 57% with a survival median age of 45.6 months.
Conclusion: Overall, our histoclinical results are consistent with those previously reported worldwide. However, the lack of follow-up data for some pNTs patients did not allow a complete and accurate survival review and emphasizes the need for a national registry for pediatric cancers, eventually with specific sections for solid tumors in children.
Keywords: Neuroblastic tumors; Morocco; Epidemiology; Incidence; Public health; Worldwide
Abbreviations: GN: Ganglioneuroma; GNB: Ganglioneuro Blastoma; I: Incidence; INPC: International Neuroblastoma Pathology Classification; INSS: International Neuroblastoma Staging System; NB: Neuroblastoma; OS: Overall survival; pNTs: Peripheral Neuroblastic Tumors
Introduction
Neuroblastoma (NB) is a malignant pediatric tumor of children under 15 years that originates from the primitive neural crest cells [1]. Worldwide, NB is one of the most common childhood cancers with leukemia, tumors of the central nervous system and lymphomas. It is also the most frequently diagnosed extra-cranial tumor in infants. NB accounts for 8-10% of all pediatric malignancies and is responsible for about 15% of childhood cancer mortality [2]. This neoplasm is characterized by diverse biological and clinical behaviors that range from spontaneous regression or differentiation into benign neoplasias, principally in children under one year of age, to very aggressive metastasis in older children [3]. As a group, these tumors define a spectrum of Peripheral Neuroblastic Tumors (pNTs), ranging from the undifferentiated NB to the mature Ganglioneuroma (GN) [4]. In Morocco, no epidemiological data regarding pNTs have been reported in either the regional cancer registry or through sporadic epidemiological studies. Through this retrospective study, we attempted to report some epidemiological and clinical characteristics of these pediatric solid tumors in the Grand Casablanca Region, the most heavily populated area of our country, over a period of 4 years and compare these findings with those reported for other countries around the world.
Methods
Data Source
Patient’s medical records were obtained from the three major public pediatric centers of Casablanca, namely the Department of Pediatric Hematology and Oncology of the 20 Août 1953 Hospital and the Departments of Pediatric Visceral Surgery and Pediatrics III of the Harouchi children’s Hospital, Casablanca, Morocco. The Ibn Rochd University Medical Center which hosts the three centers of our study is one of the oldest and largest medical centers of our country. It drains patients from the ‘Région du Grand Casablanca’ which consists of two prefectures, Casablanca and Mohammedia, and two provinces, Nouaceur and Mediouna. It is the most densely populated region in Morocco and covers an area of 1,615 km². In 2011, the population of the Grand Casablanca was estimated at 3 853 500, representing 12 % of the national population (http://www.hcp.ma/downloads/).The identification of double cases, to ensure that each case only represent a single case, is performed by the attending physician basing on the patient's full name, file number, date of birth and gender. In case of similarity, there is use of clinical data.
Inclusion Criteria and Collected Data
Children aged less than 15 years and newly diagnosed with Neuroblastoma (NB), Ganglioneuro Blastoma (GNB) and Ganglioneuroma (GN) from within the Grand Casablanca Region, during January 2007- December 2010 period, were considered. The diagnosis was based on physical exam and history, urine catecholamine studies (Vanillylmandelic Acid (VMA) and Homovanillic Acid (HVA)), blood chemistry studies, and on other tests including x-rays, ultrasound exam, CT scans of the head, chest and abdomen, a complete blood count and bone marrow aspirate. The Meta Iodo Benzyl Guanidine (MIBG) scan was done only for some patients because of its high cost.
For each case, sex, age at diagnosis (<18 months versus =18 months), INSS stage disease (I, II, III, IV and IV-S), primary and metastatic (if there) site, histology if done, relapse date (if occurred) and latest news date, were recorded. Stages I and II were combined. In order to estimate the vital status of our cohort, we called by phone all patients lost to follow-up to know their evolution.
Statistical Methods
Descriptive statistics were reported as absolute frequencies and percentages for qualitative data. Frequency differences of each variable and associations among these variables were evaluated by the Chi-square test or by Fisher’s exact test when appropriate. All analyses were performed using SPSS 16.0 Software. A p-value less than 0.05 was taken to be statistically significant. Overall Survival (OS) rates with 95% CI were calculated using Kaplan–Meier estimation. OS time was calculated from the time of diagnosis until the time of death or until time of last contact if the patient was still alive. Hazard ratios were estimated by a multivariate cox proportional hazard regression model with time dependent covariates to correct for non-proportionality. Incidence (I) was calculated by dividing the number of new cases (N) diagnosed during the 2007-2010 period in the child Population (P) (<15 years) of the Grand Casablanca Region according to the 2004 census (I = N / Px4). Ganglioneuromas, as benign tumors, were excluded from the calculation of incidence. The age groups distribution of our study population was obtained from « Le Rapport du dernier Recensement Général de la Population et de l’Habitat du Haut-Commissariat au Plan de 2004 », a 2004 report taken from the last Moroccan General Population and Housing Census. (http://www.hcp.ma/Recensement-general-de-la-population-et-de-l-habitat-2004_a633.html). The incidence was standardized on world child population (http://social.un.org/).
Results
Age and Stage
There were 82 patient’s clinical files available for study between January 2007 and December 2010. The youngest patient was 5 days old, the oldest was 13 years old with median age of 38.6 months at diagnosis. Table 1 shows data concerning sex ratio, median age and distribution by stage of NBs in our study compared to some countries around the world. There were 49 (59.8 %) boys and 33 girls (40.2 %), M: F = 1.5. Approximately two thirds of patients (68.3%) were aged 18 months or older while 31.7% were infants (less than 18 months). About 79.3% (65 cases) of the children were younger than five years.
UK
France
Hungary
Germany
Austria
USA
aSouthern Africa
Thailand
China
Our study
Sex ratio M/F
1.12
1.14
1.4
1.24
1.22
1.2
0.9
1.7
1.3
1.5
Median age (months)
24
24
25.8
17.3
14.5
18
18
34.8
48
38.6
Age at diagnosis
< 1 year
25.9
38.5
30
42.4
42
ND
ND
11
16.3
23.2
1-4 years
59.5
47.9
53
46.9
43
ND
58.1
56.1
5-9 years
12.8
11.4
12
8.5
10
21.5
18.3
10-14 years
1.9
2.2
5
2.2
4
4.1
2.4
Stages (%)
I/II and IV-S
19.5
34.2
33
33.2
35
b
29
11.3
18.4
29.2
III and IV
75.7
65.4
65
62.9
65
71
85.5
81.6
70.8
Not classified
4.7
0.5
2
3.9
0
0
3.2
0
0
References
[5]
[5]
[6]
[5]
[5]
[12]
[13]
[14]
[15]
aFor Southern Africa, tumors were distributed according to the Evans classification system
bFor this American study, the percentages were calculated for INSS stages I, II, III and IV-S (54%) versus INSS stage IV (46%).
ND: Not Determined
Table 1: Percentages of sex-ratios, median ages and INSS stages of our study compared to those of some countries in the world.
Distribution according to International Neuroblastoma Staging System (INSS) was: stages I/II (n=18; 22%), stage III (n=15; 18.3%), stage IV (n= 43; 52.4%) and stage IV-S (n=6; 7.3%). Patients with low grade disease (INSS stages I/II and IV-S) accounted for 29.2 % of cases whereas 70.8 % showed high grade disease (INSS stages III and IV). Table 2 shows the distribution of cases according to age, sex and INSS stages.
Age
Stage
Male(%)
Female(%)
< 1 year
I/II and IV-S
6 (31.6)
5 (26.3)
III and IV
7 (36.9)
1 (5.2)
1-4 years
I/II
2 (4.3)
5 (10.9)
III and IV
21 (45.7)
18 (39.1)
5-9 years
I/II
3 (20)
2 (13.3)
III and IV
8 (53.4)
2 (13.3)
10-14 years
I/II
1 (50)
0 (0)
III and IV
1 (50)
0 (0)
Table 2: The percentage of Peripheral Neuroblastic Tumors cases by age, INSS stage and sex in Morocco.
Incidence
In our study, the annual incidence of neuroblastic tumors was 14.2 cases per 1.000.000 children (95% CI: 11.1-17.3) and the age-standardized incidence rate was 14.5 per million children per year (95% CI: 11.3-17.7). Age-specific and age-standardized incidence rates are shown in Table 3. Three age groups were considered: 0-4 years, 5-9 years and 10-14 years.
Histopathology
The histological examination was performed in only 41/82 (50%) of all cases. All pNTs were first classified into four categories: NB (Schwannian stroma-poor); GNB intermixed (GNBi/ Schwannian stroma-rich); GN (Schwannian stroma-dominant); and GNB nodular (GNBn/ composite: Schwannian stroma-rich/stroma-dominant and stroma-poor). The distribution of tumor subtypes is shown in Table 4.
Subtypes
n = 41 (%)
Undifferentiated neuroblastoma
9 (22)
Poorly differentiated neuroblastoma
12 (29.3)
Differentiating neuroblastoma
4 (9.7)
Ganglioneuroblastoma intermixed
5 (12.2)
Ganglioneuroblastoma nodular
8 (19.5)
Ganglioneuroma
3 (7.3)
Table 4: The distribution of pNTs subtypes in our study population.
According to the International Neuroblastoma Pathology Committee (INPC) classification (based on the modified Shimada grading), 24 pNTs (58.5%) showed unfavorable histology whereas 17 (41.5%) were classified into favorable histology.
Tumor Sites
As shown in Table 5A, the primary site of the tumor was mainly abdominal in 84.1% of cases predominantly at the adrenal (63.8%). Thoracic, pelvic, cervical and intraspinous locations were also identified in 8.6%, 3.7%, 1.2% and 1.2% of cases respectively. In 1.2% of cases, the primary site of disease was not detected.
A
UK
France
Turkey
Southern Africa
Our study
Primary sites (%)
Abdomen
74
70
72.2
75
84.1
Thorax
13
17
14.9
15
8.6
Pelvis
2
2
3.8
5
3.7
Others
10
6
9.1
5
2.4
Unknown primary
2
5
0
0
1.2
References
[11]
[17]
[18]
[13]
Table 5: Distribution of patients’ primary (A) and metastatic (B) tumors sites.
B
China
France
Our study
Metastatic sites (%)
Bone marrow
26.5
20
38.1
Bone
20.4
13
22.6
Lymph nodes
NS
28
21.4
Liver
NS
22
14.3
Lung
NS
3
3.6
Skin
NS
1.2
3.6
References
[15]
[17]
NS: Not Specified
Table 5 off 1:
Tumors were widespread at diagnosis in 61% of cases whereas 39% did not extend beyond the primary site. Metastases were found in bone marrow (38.1%), bone (22.6%), lymph nodes (21.4%), liver (14.3%), kidney (4.8%), skin (3.6 %), lung (3.6%), brain (2.4%) and pancreas (1.2%) (Table 5B).
Follow-up
One child had no follow-up at all and was excluded from the survival study. As shown in Figure 1, OS at 24 months and 36 months was estimated to be around 65% and 57% respectively. The survival median age of the cohort was 45.6 months.
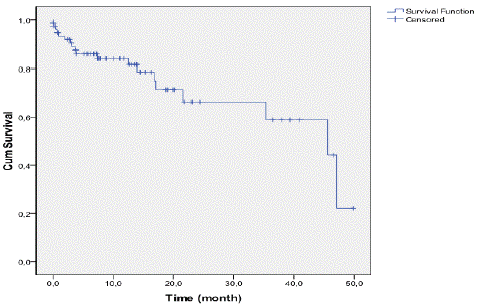
Figure 1: Overall survival curve for the entire cohort of patients.
The four relapsed cases observed in our study have occurred among children older than 18 months who had advanced INSS stages (1 stage III and 3 stage IV). Three of them died and the fourth patient was lost to follow-up.
Figure 2 shows the overall survival of children according to age (a), INSS stage (b), primary site (c) and metastasis (d): We noticed a better long-term OS in children aged less than 18 months, at low stage, in the nonabdominal primary site and in patients without metastases, however, these differences were not statistically significant and the curves crossed: during the first months of childhood, OS seemed better for the prognostically unfavorable groups (=18 months, high INSS stage, abdominal primary site and metastatic disease) probably due to the high number of patients lost to follow-up during this period. In the multivariate Cox proportional hazard regression model with time-dependent covariates, the non-abdominal site had a higher risk (HR 9.29, p=0.028). This effect decreases with time. The age, stage and metastasis were not significantly associated with survival (Table 6).
HR
CI95%
p
TD Covariates HR (p)
Age
=18months versus <18 months
0.25
0.02-2.80
0.259
1.06 (0.668)
Stage
III, IV versus I,II, IVs
2.72
0.27-26.95
0.393
0.88 (0.353)
Site
Non abdominal versus abdominal
9.29
1.28-67.61
0.028
0.39 (<0.001)
Metastasis
Presence versus absence
1.77
0.24-13.05
0.576
1.11 (0.406)
HR: Hazard Ratio
CI: Confidence Interval
TD Covariates: Time-Dependent Covariates
Table 6: Estimated hazard ratios from multivariate Cox model with time-dependent covariates.
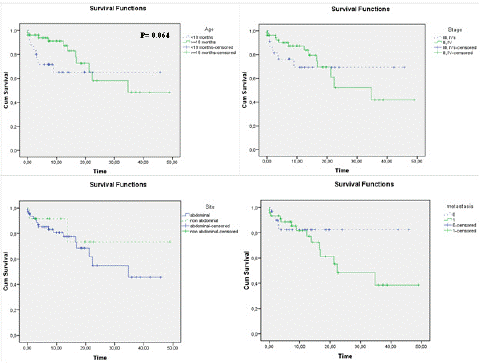
Figure 2: Overall survival curves in relation to: (a) age, (b) INSS stage, (c) primary site and (d) presence /absence of metastases.
Discussion
The last regional cancer registry in Morocco has been published in 2018 [7] and did not present specific data concerning neuroblastoma although this is one of the most common childhood cancers and responsible for 15% of cancer mortality in children [2]. To our knowledge, there was no statistical report regarding the epidemiology of NB in Morocco in the past. For this purpose, we retrospectively analyzed the epidemiological data, the clinical parameters and the histological features in 82 children newly diagnosed for peripheral neuroblastic tumors from January 2007 to December 2010 in the three major care referral centers for diagnosis and treatment of pediatric oncology in Casablanca, Morocco.
The age-standardized incidence rate reported in our study 14.5 (11.3-17.7) was slightly higher compared to that from Austria 11.7 (9.0–14.5), France 12.5 (11.5–13.5), Germany 11.4 (10.4–12.4), UK 10.1 (9.2–11.0) and Hungary 12.8 (10.6–15.0). Infant age group (< 1 year) could not be distinguished due to the lack of data on the population at risk, so we have not been able to compare the incidence rates of the < 1 year and 1-4 years age groups with those of other statistical surveys. We found that among the 5-9 years group, the NB and GNB incidence rate in our study: 8.3 (4.1-12.5) was twice higher than in UK: 3.6 (2.7-4.4), France: 3.7 (2.8-4.5), Germany: 2.7 (1.9-3.5), Austria: 3.1 (1.2-6.4) and Hungary: 4.2 (2.6-5.8).
The low incidence rate observed in our study among the 0-4 years age group may reflect a delayed diagnosis of NB in Morocco during the early years of childhood which, in turn, could explain the high incidence observed from the fifth year of childhood (Table 3). A previous report from the Automated Childhood Cancer Information System (ACCIS), a collaborative project of 80 population-based cancer registries in 35 European countries, had studied the variation in incidence for age-groups 0, 1–4, 5–9, and 10–14 years. It showed that, among European children diagnosed during 1978–1997, the incidence of NB was highest in the first year of life; incidence rates declined considerably thereafter and cases were rare beyond the 10th birthday [8].
UK
France
Germany
Austria
Hungary
Our study
Age at diagnosis
< 1 year
33.7 (27.8–39.5)
63.7 (55.7–71.8)
61.6 (53.2–69.9)
65.8 (44.1–94.5)
60.9 (40.6–81.1)
1-4 years
19.9 (17.6–22.2)
19.9 (17.6–22.1)
18.0 (15.7–20.3)
17.0 (11.4–24.2)
25.5 (19.8–31.2)
0-4 years
34.2 (25.8-42.6)
5-9 years
3.6 (2.7–4.4)
3.7 (2.8–4.5)
2.7 (1.9–3.5)
3.1 (1.2–6.4)
4.2 (2.6–5.8)
8.3 (4.1-12.5)
10-14 years
0.5 (0.2–0.9)
0.7 (0.3–1.1)
0.7 (0.3–1.2)
1.3 (0.3–3.9)
1.7 (0.8–2.4)
0.52 (0.0-1.5)
Age-standardised
10.1 (9.2–11.0)
12.5 (11.5–13.5)
11.4 (10.4–12.4)
11.7 (9.0–14.5)
12.8 (10.6–15.0)
14.5 (11.3-17.7)
Table 3: Age-specific and directly age-standardized (world population) incidence rates (per million) (95% CI) for neuroblastoma and ganglioneuroblastoma in our study (2007–2010) and in some European countries [5,6].
The comparison of our results with those previously reported worldwide has been made difficult by the non-standardization of threshold values and selection criteria adopted by different epidemiological studies. Thus, with regard to age, for example, some studies continue to adopt the threshold of 12 months, while others preferred to switch to the age-cutoff of 18 months which was recently recommended because it seems to reflect the biology of neuroblastic tumors better than the traditional 12-month cutoff [9]. Furthermore, staging systems also differed from one study to another: while older studies have classified the tumors according to the Evans classification, the recent and current studies adopt either the INSS or the more recent classification of the International Neuroblastoma Risk Group Staging System (INRGSS) [10].
Table 1 shows data concerning sex ratio, median age and distribution by stage of NBs in our study compared to some countries around the world. Our data indicated that, in the largest and the most populated region in Morocco, approximately 20.5 new cases of NB were diagnosed each year. Our study showed a slightly higher sex ratio (M/F= 1.5) compared to Southern Africa: M/F= 0.9). Regarding sex ratios reported in studies conducted in some countries in the world, UK: M/F = 1.12, France: M/F = 1.14, Hungary (M/F = 1.4), Germany (M/F = 1.24), Austria (M/F = 1.22), USA (M/F = 1.2) and China (M/F = 1.3), we noted, as in our work, an obvious male predominance also reported by Spix et al. through the European ACCIS study [8]. The cause of this gender-related difference is still not explained.
Variation in the efficiency of health-care systems remains the most likely explanation of our findings concerning the mean age at diagnosis since we could note that the highest values of median ages at diagnosis were observed in China, Thailand and Morocco whereas UK, France, USA have lower median ages values.
Diagnosis at an earlier age thanks to efficient diagnostic methods could explain the variation observed between Europe (UK, France, Hungary, Germany, Austria) and Asia (Thailand and China). Through our study, it appears that Africa (Morocco and South Africa) is positioned between the European countries (except UK) on one hand and Asia on the other hand. Regarding UK, it seems that the efficiency of NB diagnosis was much lower than in mainland Europe and other advanced countries because of less rigorous routine infant health surveillance [5,11].
We have already published a previous manuscript evaluating MYCN protooncogene amplification status by FISH and describing, among others, age and INSS staging of 36 pNTs patients that had been subsequently included in the current work [16]. The percentages resulting from the present investigation were overall in line with those previously reported with a rate of 68.3 % of children =18 months (current study) versus 63.9 % (previous study) and a rate of 70.8% of INSS stages III, IV (current study) versus 61.1% (previous study).
Late diagnosis of these tumors in Morocco may explain the observed high percentage of advanced and poor prognosis INSS stages. These differences could indicate a low capacity of the Moroccan health system to manage such complex diseases and we can suggest that in Morocco, diagnosis of neuroblastic tumors is delayed because of the low socioeconomic status of the patients who turn to public hospital and the weakness of health insurance coverage. Moreover, cases may be missed for diagnosis because of absence of rigorous child health checks. Overall, these variances in INSS stages distribution may reflect the different pediatric health-care systems in countries around the world.
As previously reported by other studies and illustrated in Table 5 A, the most common primary site of NB is the abdomen (with a predominance at the adrenal location); less common sites of origin are the thorax and pelvis, while primary site is rarely not identified. In agreement with the previously published data, we found that more than 60% of the tumors extended beyond the primary site of disease at the time of diagnosis, the most common metastatic sites being bone marrow, bone, lymph nodes and liver (Table 5 B). We noted a relatively high frequency of brain metastases (2.4%) compared to that reported in the literature (<1%) [1,2].
The multivariate Cox proportional hazard regression model with time-dependent covariates demonstrated a statistically significant association between tumor site and overall survival, since the non-abdominal site presented a higher risk (p=0.028). However, this effect decreased with time probably since a lot of patients were lost to follow-up.
On the other hand, the high level of metastatic cases means that the medical care for NB in Moroccan hospitals should be much improved. There is need for refining the diagnosis, staging system and risk stratification. This improvement should also lead to an earlier diagnosis of these tumors in our country.
We realize that this first study contains limitations relating to weakness of follow-up data and, unfortunately, data on treatment were not recorded. On the other hand, it would be more appropriate to consider the age cutoff of 18 months for infants versus older age groups. This and the use of image-defined risk factors (IDRFs), would allow the adoption of the new staging (INRGSS) and risk classification (INRG) of neuroblastomas in our country. Furthermore, the assessment of additional detailed genetic studies, such as ploidy, 11q LOH (loss of heterozygosity), 1pLOH and TrkA expression will help to refine risk stratification.
Conclusion
The creation of regional registers and subsequently of a national registry of childhood cancer in Morocco remains an urgent need for better understanding and management of this problem. Moreover, the search for risk factors, including environmental and genetic factors, will guide health policy in Morocco to appropriate control and treatment strategies in order to improve survival of children with this neoplasm.
Author Statements
Acknowledgements
We thank all the staff who supported our study.
References
- Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018; 372: 211–221.
- Swift CC, Eklund MJ, Kraveka JM, Alazraki AL. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics. 2018; 38: 566–580.
- Aygun N. Biological and Genetic Features of Neuroblastoma and Their Clinical Importance. Curr. Pediatr. Rev. 2018; 14: 73–90.
- Nakazawa A. Biological categories of neuroblastoma based on the international neuroblastoma pathology classification for treatment stratification. Pathol Int. 2021; 71: 232-244.
- Powell JE, Estève J, Mann JR, Parker L, Frappaz D, Michaelis J, et al. Neuroblastoma in Europe: differences in the pattern of disease in the UK. SENSE. Study group for the Evaluation of Neuroblastoma Screening in Europe. Lancet. 1998; 352: 682-687.
- Nyári TA, Kajtár P, Parker L. Neuroblastoma in hungary. Eur J Cancer. 2006; 42: 2350-2354.
- Link/ URL: Registre des Cancers de la Région du Grand Casablanca (RCGC). Rapport d’incidence. 2013-2017.
- Spix C, Pastore G, Sankila R, Stiller CA, Steliarova-Foucher E. Neuroblastoma incidence and survival in European children (1978–1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006; 42: 2081-2091.
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, et al. Evidence for an Age Cutoff Greater Than 365 Days for Neuroblastoma Risk Group Stratification in the Children’s Oncology Group. J Clin Oncol. 2005; 3: 6459-6465.
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009; 27: 289-297.
- Salim A, Mullassery D, Pizer B, McDowell HP, Losty PD. Neuroblastoma: a 20-year experience in a UK regional centre. Pediatr Blood Cancer. 2011; 57: 1254-1260.
- Henderson TO, Bhatia S, Pinto N, London WB, McGrady P, Crotty C, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children's Oncology Group study. J Clin Oncol 2011; 29: 76-82.
- Hesseling PB, Ankone K, Wessels G, Schneider JW, Du Plessis L, Moore S. Neuroblastoma in southern Africa: epidemiological features, prognostic factors and outcome. Ann Trop Paediatr 1999; 19: 357-363.
- Shuangshoti S, Shuangshoti S, Nuchprayoon I, Kanjanapongkul S, Marrano P, Irwin MS, et al. Natural course of low risk neuroblastoma. Pediatr Blood Cancer. 2012; 58: 690-694.
- Li K, Dong K, Gao J, Yao W, Xiao X, Zheng S. Neuroblastoma management in Chinese children. J Invest Surg. 2012; 25: 86-92.
- Tabyaoui I, Tahiri Jouti N, Serhier Z, El Maani K, Cherkaoui S, Al Zemmouri M, et al. High incidence of MYCN amplification in a Moroccan series of neuroblastic tumors: comparison to current biological data. Diagn Mol Pathol. 2013; 22: 112-118.
- d'Andon A, Pein F, Valteau-Couanet D, Couanet D, Hartmann O. Le Neuroblastome 2004. www.igr.fr/pdf.
- Aydn GB, Kutluk MT, Yalçn B, Büyükpamukçu M, Kale G, Varan A, et al. Neuroblastoma in Turkish children: experience of a single center. J Pediatr Hematol Oncol. 2009; 31: 471-480.