
Review Article
Austin J Public Health Epidemiol. 2022; 9(1): 1121.
Impact of Anthelmintic Treatment on the Burden of Helminth Infections in Primary Schoolchildren in Biyela Health Zone in Kinshasa, Democratic Republic of the Congo
Linsuke S1,2*, Matangila J3, Doua JY2, Lutumba P3 and Van Geertruyden J-P2
¹Department of Epidemiology, National Institute of Biomedical Research (INRB), Kinshasa, Democratic Republic of the Congo
²Global Health Institute, University of Antwerp, Antwerp, Belgium
³Department of Tropical Medicine, University of Kinshasa, Kinshasa, Democratic Republic of the Congo
*Corresponding author: Linsuke S, Department of Epidemiology, National Institute of Biomedical Research (INRB), Kinshasa, Democratic Republic of the Congo
Received: February 11, 2022; Accepted: April 06, 2022; Published: April 13, 2022
Abstract
Background: The study evaluated the impact of anthilminthic treatment, given as non-investigational drugs, on helminth infections, anaemia, and hemoglobin (Hb) level in the context of a malaria clinical trial in which Sulfadoxine Pyrimethamine (SP) and SP+Piperaquine (PQ) were also evaluated as an intermittent preventive treatment strategy in schoolchildren in the Democratic Republic of Congo (DRC).
Methods: In a nested cohort study, 616 malaria asymptomatic children attending primary schools of Biyela health area were enrolled and follow-up from November 2012 to November 2013. The control group for the nested study was the negative on helminth infections. They received one dose of PZQ and ALB at baseline and then 2 doses of ALB at 4 months intervals. During the 12-months of follow-up, stool and urine samples were collected for helminth infections and finger prick blood for Hb level. Paired test analyses were used to compare the status before and after treatment, and confounding variables for Hb level were tested by multiple linear regression analysis.
Results: At baseline, the prevalence of helminth infections and anaemia were 39.2% (95% CI: 34.7-43.7), and 41.8% (95% CI: 37.3-46.3), respectively. Mean Hb level was 11.6g/dl (SD±1.3). After 12-months post-anthelminthic treatment, the prevalence of helminth infections reduced to 7.2% (p<0.001). There was no change in Hb level and anaemia in the control which received only the anthelminthic drug (p=1.00 and p=0.26, respectively) at 12-months postintervention, compared to those who received active antimalarial SP (p=0.02 and p=0.09, respectively) and PQ (p=0.01 and p<0.001, respectively). Similarly, no difference in Hb level was observed among the infected and uninfected schoolchildren with helminths at 12-months after anthelminthic treatment.
Conclusion: These findings suggest that anthelminthic treatment reduces significantly the prevalence of helminth infections. There was no impact on anaemia and Hb level due to the use of anthelmintic drugs.
Keywords: Helminth; Malaria; Anthelminthic treatment; Hemoglobin level; Schoolchildren; The Democratic Republic of the Congo
Abbreviations
SCH: Schistosoma spp; STH: Soil-Transmitted Helminths; SSA: Sub-Saharan Africa; SAC: School-Aged Children; PZQ: Praziquantel; ALB: Albendazole; MBZ: Mebendazole; DRC: The Democratic Republic Of The Congo; MDA: Mass Drug Administration; RCT: Randomized Clinical Trial; IPTsc: Intermittent Preventive Treatment in Schoolchildren; SP: Sulfadoxine Pyrimethamine; PQ: Piperaquine; Hb: Hemoglobin
Background
In 2019, 236.6 million and 1.5 billion people were worldwide chronically infected with helminth infections caused by Schistosoma spp (SCH) and soil-transmitted helminths (STH) respectively [1]. Most cases were found in sub-Saharan Africa (SSA). The burden of disease caused by these helminth infections is estimated to 1,627,844 and 2,748,420 disability-adjusted life years for SCH and STH respectively in 2019 [2].
SCH and STH are among the most frequent health problems affecting school-aged children (SAC) living in tropical areas, where low hygienic and environmental conditions allow their transmission [3-5]. Approximately 1282 million SAC are estimated to be at risk for SCH and 7464 million for STH [1]. SAC is an important highrisk group for infections because they are traditionally responsible for water-related household chores in endemic countries, like spending free time swimming, and living in the poorest sanitation environment. Chronic (co-) infection with these helminths has health consequences such as anaemia, nutritional troubles, alteration of growth, impaired cognitive abilities, and irreversible consequences in adulthood [6-8]. Concomitant infections with other parasites such as P. falciparum are frequently observed and may have additional effects or worsen the health status [9,10]. Therefore, preventive and curative interventions are needed in SAC.
According to the World Health Organization (WHO), the main aim of SCH and STH control is to reduce or prevent related morbidity and transmission [11]. The control strategy is based on a periodic deworming of the at-risk population in the endemic area using praziquantel (PZQ) 40mg/Kg and albendazole (ALB) 400mg or mebendazole (MBZ) 500mg, respectively for SCH and STH control. This approach is used in many national control programs against SCH and STH across SSA with positive effects on health status [12,13].
Democratic Republic of Congo (DRC) is one of the countries in SSA most affected by SCH and STH (A. lumbricoides, T. trichiuria, hookworm) infections [3,14]. Recent data in DRC showed a high prevalence of these parasitic infections, especially among SAC [15-18]. The Ministry of Health of DRC throughout the National Schistosomiasis and Intestinal Parasitic Infections Control Program has adopted the periodic mass drug administration (MDA) of PZQ and ALB. However, this strategy has only been implemented in children attending schools, and little is known about the impact of this school-based deworming program on children’s health.
This nested study aimed to assess the added value of anthelminthic while schoolchildren received in a randomized trial design whether antimalarial drugs in intermittent preventive treatment (IPT) strategy using either SP or SP/PQ. We assessed and compared the baseline data with the 12-months follow-up data regarding the helminth infections, anaemia, and difference in hemoglobin (Hb) level.
Methodology
Ethics statement
This study is a secondary analysis of a RCT evaluating the efficacy and safety of IPTsc with Sulfadoxine Pyrimethamine (SP) and SP+Piperaquine (PQ) on anaemia and malaria morbidity in Congolese schoolchildren. Trial Registration Number: NCT01722539; https:// clinicaltrials.gov/ct2/show/NCT01722539 [19]. Ethical clearance was sought and obtained from the Ethical Committee of the School of Public Health, Kinshasa; DRC, and the University of Antwerpen, Belgium before the launch of the study. Written informed consent was also obtained from parents or legal guardians of all children before their enrollment [20].
Study area and period
The study was conducted from November 2012 to November 2013 in Mokali health area of Biyela Health Zone in Kinshasa, RDC. This urban-rural health zone is one of the health zones of Kinshasa endemic for SCH and STH [17,18] but no MDA was ever implemented. The administered population of Biyela health zone was 182,421 inhabitants distributed over nine health areas, and approximately 15% of this population lives in Mokali health area. The climate is tropical with a rainy season from September to May, followed by a short dry season. Biyela’s population has no access to tap water. The three rivers that cross the area: Mokali, Mango, and Nsanga represent the main source of water for household duties and children’s baths. Moreover, most households do not have toilets leading to poor sanitary conditions (archive report of Health Zone, unpublished data).
Study design and procedures
This was a nested cohort study from schoolchildren enrolled in the RCT study. This study enrolled malaria asymptomatic schoolchildren from the first to 5th primary school year in two schools (EP Boyambi and EP Likabo) in Mokali health area and randomly allocated them to three treatment arms: SP given alone, SP combined with PQ, and the control group without malaria prevention. Details on procedures and results of this RCT are largely described by Matangila et al in 2019 [20].
Study subjects
All participants were enrolled irrespective of helminth infections status.
Helminth infections assessment
Stool and urine samples were collected at the enrollment and 12-months later for STH and SCH diagnosis. Blood samples were collected at baseline, at months 4, 7, and 12, for for Hb assessment. Plasmodium detection was done at the baseline and month 12 [19].
Stool samples were processed using the Kato-Katz technique (2x25mg) for the presence of intestinal helminth infections [21]. Two slides were performed and examined microscopically within 24h of preparation by two different technicians. Results were expressed as eggs per gram of feces (epg/gr). To obtain helminth infections intensities, the number of eggs found on duplicate Kato Katz slide was multiplied by factor 20, and intensities were classified into low, moderate, and heavy infection per WHO criteria [22].
Urine samples were tested using urine reagent strips (Combur7 Test®, Roche Diagnostics GmbH, Manheim, Germany) to the detected presence of microscopic hematuria. The results were recorded semiquantitatively: negative, positive with (+), (++) and (+++). Moreover, all urine samples positive for urine reagent strips were processed by the centrifugation technique for the detection of S. haematobium eggs in urine [23]. The intensity of infection was estimated according to the number of eggs per 10ml of urine and classifying per WHO criteria [22].
Helminth infections were defined as a presence of any helminth either in the stool or urine samples.
Measuring Hb level and determination of anaemia
Blood samples were obtained from each child by the finger prick and the Hb level was determined using the portable photometer (HemoControl®, EKF Diagnostics, Germany) per manufacturer’s instructions [24]. Anaemia was defined by Hb concentration <11g/dl for children aged under 5 years, <11.5g/dl for children 5-11.9 years, and <12g/dl for children aged 12-14.9 years [25].
Helminth treatment
After sample collection for baseline assessment, all participants were treated twice with PZQ (40mg/kg) and ALB (400mg) per WHO protocol [22]. The first dose of PZQ was given at baseline and a second at the 12-month follow-up survey. While for ALB, the first dose was given at baseline and 2 consecutive doses were given at an interval of four months after baseline. PZQ and ALB used in this study were supplied by the IDA Foundation, Amsterdam, Netherlands.
Data management and statistical analysis
Survey data were double entered in Epi-Info 3.5.1 version (Centers for Disease Control and Prevention, Atlanta, USA) and imported into StataIC 11.1 software (StataCorp., College Station, TX, USA) for statistical analysis.
Statistical analysis for the impact of anti-helminth treatment on helminth infections was restricted to schoolchildren who provided a stool and urine samples at month 12. While analysis of the impact of anti-helminth treatment on Hb level or anaemia was done on all schoolchildren who provided a blood sample at month 12 irrespective the presence of a stool and urine samples after 12-months (Figure 1). To control for the influence of antimalarial treatment on the Hb level, we stratified the groups randomized to an active antimalarial (SP, SP+PQ) and the control group not receiving an antimalarial. A descriptive analysis summarized the data. Paired tests analysis compared the prevalence of helminth infections, anaemia, and the mean Hb level before and 12-months after treatment delivery. Likewise, a stratified analysis was done to evaluate the effect of treatment in infected and uninfected subjects at baseline. The influence of the antimalarial intervention on the boost of the Hb level at 12-months post-treatment was explored using multiple linear regression analysis. The significant level was set at a P-value <0.05.
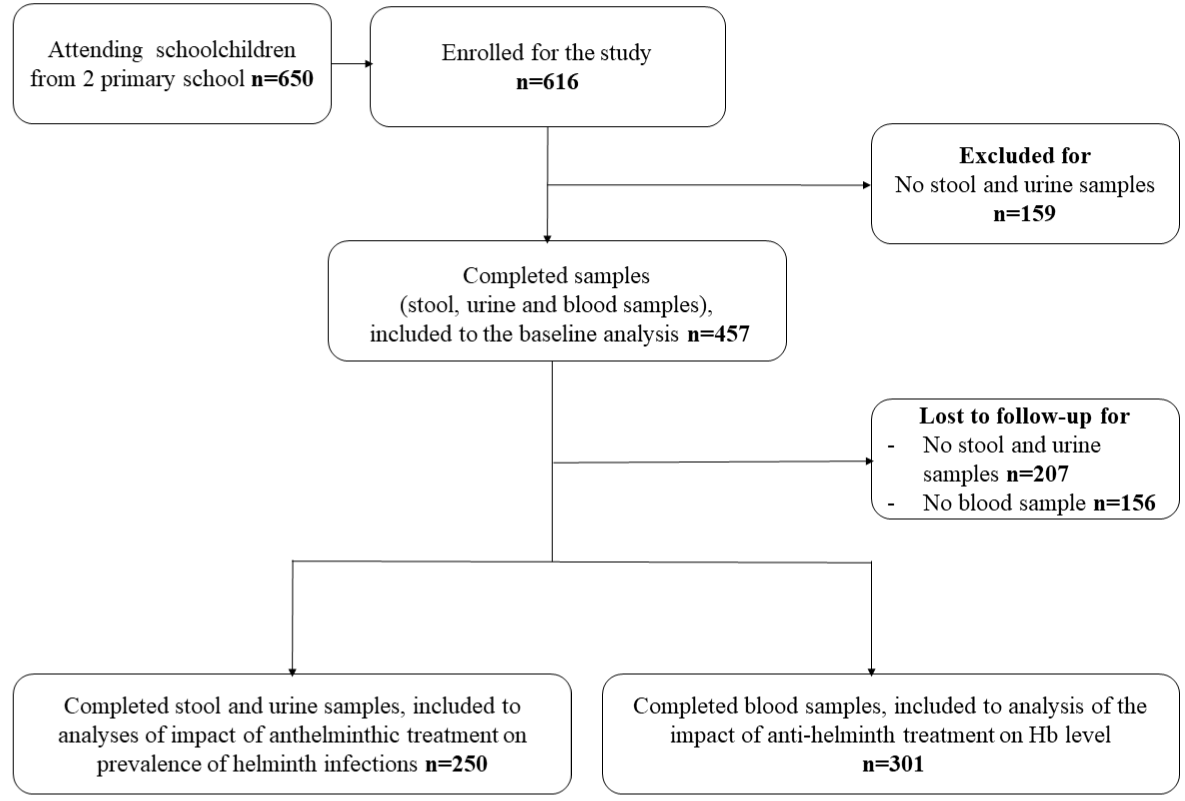
Figure 1: Flow-diagram of the study showing 457 participants included in the study.
Results
Baseline characteristics of the study population
Overall, 616 schoolchildren from the two primary schools were enrolled in the RCT study. At baseline, out of the 616 enrolled children, 159 (25.8%) children did not give the stool and urine samples while 457 (74.2%) provided stool and urine samples, and were tested for helminth infections (Table 1). The median age, the proportion by sex, Overall, 616 schoolchildren from the two primary schools were enrolled in the RCT study. At baseline, out of the 616 enrolled children, 159 (25.8%) children did not give the stool and urine samples while 457 (74.2%) provided stool and urine samples, and were tested for helminth infections (Table 1). The median age, the proportion by sex,
Variables
All
Helminth infections
Positive
Negative
n
% (95% CI)
n
% (95% CI)
n
% (95% CI)
Total
457
179
39.2 (34.7-43.7)
378
60.8 (56.3-65.3)
Demographic characteristics
Median age, years (IQR)
8 (7-9)
8 (7-9)
8 (6-8)
Age 4-7 years
208
45.5 (40.9-50.1)
74
41.3 (34.1-48.6)
234
48.2 (42.3-54.1)
Age 8-13 years
249
54.5 (49.9-59.1)
105
58.7 (51.4-65.9)
144
51.8 (45.9-57.7)
Male
242
53.0 (48.4-57.5)
101
56.4 (49.1-63.7)
141
50.7 (44.8-56.6)
Malaria infection
87
19.0 (15.4-22.7)
36
20.1 (14.2-26.0)
51
18.3 (13.8-22.9)
Mean Hb, g/dl±SD
11.6±1.3
11.5±1.2
11.6±1.3
Anaemia
191
41.8 (37.3-46.3)
76
42.5 (35.2-49.7)
115
41.4 (35.6-47.2)
Table 1: Baseline characteristics of schoolchildren in Kinshasa, Democratic Republic of the Congo.
Changes in the prevalence of helminth infections
Over 12-months post-treatment, 301 persons provided samples: 249 (54.5%) provided stool, urine and blood samples while 250 (54.7%) provided stool and urine, and 301 (65.9%) provided blood samples (Figure 1). General characteristics of participants lost to follow-up were not different from those who were present 12 months after treatment delivery (data not shown).
There was a decrease in the prevalence of helminth infections 12 months post-treatment from 37.6% at baseline to 7.2% (p<0.001) and this decrease was similar in all malaria intervention groups (Table 2). At 12 months, assuming a total helminth clearance, the cumulative incidence was similar in infected and non-helminth infected [8 (8.5%) vs 10 (6.4%); p=0.62].
Variables
All participants (N=250)
No malaria intervention group (N=83)
SP group (N=93)
SP/PQ group (N=74)
Baseline
12 months
RR (95% CI)
P-value
Baseline
12 months
RR (95% CI)
P-value
Baseline
12 months
RR (95% CI)
P-value
Baseline
12 months
RR (95% CI)
P-value
No. (%)
No. (%)
No. (%)
No. (%)
No. (%)
No. (%)
No. (%)
No. (%)
Helminth infections
94 (37.6)
18 (7.2)
0.19 (0.12-0.31)
<0.001*
28 (33.7)
6 (7.2%)
0.2 (0.1-0.5)
0.001*
33 (35.5)
3 (3.2%)
0.09 (0.03-0.27)
<0.001*
65 (87.8)
9 (12.2)
0.27 (0.14-0.54)
0.001*
All participants (N=301)
No malaria intervention group (N=104)
SP group (N=109)
SP/PQ group (N=88)
Anemia
120 (39.9)
93 (30.9)
0.77 (0.65-0.93)
0.006*
40 (38.5)
40 (38.5)
1.0 (0.75-1.3)
1
44 (40.4)
31 (28.4)
0.7 (0.5-0.96)
0.02*
36 (40.9)
22 (25)
0.6 (0.4-0.9)
0.006*
Helminth infection
47/119 (39.2)
35/119 (29.4)
0.74 (0.55-1.01)
0.06
16/39 (41.0)
13/39 (33)
0.8 (0.5-1.4)
0.44
29 (41.4)
12 (30.8)
0.8 (0.4-1.5)
0.47
20 (42.6)
12 (25.5)
0.6 (0.4-0.97)
0.03*
Non-Helminth infection
73/182 (40.1)
58/182 (31.9)
0.79 (0.64-0.99)
0.04*
24/65 (36.9)
27/65 (41.5)
1.1 (0.8-1.6)
0.49
15 (38.5)
19 (27.1)
0.7 (0.5-0.9)
0.01*
16 (44.4)
10 (24.4)
0.6 (0.4-1.0)
0.06
Mean (95% CI)
Mean (95% CI)
MD (95% CI)
P-value
Mean (95% CI)
Mean (95% CI)
MD (95% CI)
P-value
Mean (95% CI)
Mean (95% CI)
MD (95% CI)
P-value
Mean (95% CI)
Mean (95% CI)
MD (95% CI)
P-value
Hb level
11.6 (11.5-11.8)
11.9 (11.8-12.1)
0.3 (0.15-0.45)
0.001*
11.7 (11.4-11.9)
11.8 (11.6-12.0)
0.2 (-0.1-0.4)
0.26
11.8 (11.5-12.0)
11.9 (11.7-12.1)
0.2 (-0.0-0.45)
0.09
11.4 (11.2-11.7)
12.0 (11.8-12.3)
0.6 (0.3-0.8)
<0.001*
Helminth infection
11.6 (11.4-11.8)
12.0 (11.8-12.2)
0.4 (0.1-0.6)
0.0015*
11.7 (11.4-12.0)
12.0 (11.6-12.3)
0.3 (-0.2-0.8)
0.18
11.6 (11.2-11.9)
11.8 (11.4-12.2)
0.2 (-0.2-0.7)
0.27
11.5 (11.1-11.9)
12.1 (11.7-12.4)
0.6 (0.3-0.9)
0.0005*
Non-Helminth infection
11.7 (11.5-11.9)
11.9 (11.7-12.1)
0.2 (0.1-0.4)
0.0126*
11.7 (11.3-12.0)
11.7 (11.5-12.0)
0.1 (-0.3-0.4)
0.71
11.9 (11.6-12.1)
12.0 (11.8-12.3)
0.2 (-0.1-0.5)
0.19
11.4 (11.0-11.8)
12.0 (11.6-12.4)
0.6 (0.2-1.0)
0.0042*
P-value = mc Nemar test or paired t-test.
*Significant at <0.05.
Table 2: Stratified analysis of the impact of anthelminthic treatment on helminths infection, Hb level, and anaemia on schoolchildren stratified by malarial IPT intervention.
Changes in the proportion of anaemia, and Hb level
At 12-months post-intervention, there was no change in anaemia in the control group, which received only the anthelminthic drug (p=1.00) and mean Hb increased by 0.2g/dl (CI96: -0.1-0.4; p=0.26). In those who received active antimalarial, we observed no additional impact of helminth treatment in mean Hb levels or anaemia between helminth infected and uninfected schoolchildren at 12-months follow up (Table 2). The multivariate regression model (Table 3) showed that the mean Hb level increased with antimalarial treatment in the IPTsc intervention with the SP+PQ (p=0.01) and malaria infection at baseline (p<0.001). Other factors were not found to contribute to improving the mean Hb level. While all participants received anthelmintic drugs, we found no interaction between malaria drugs and anthelmintic. In the non-malaria intervention group, despite the treatment with anthelminthic drugs, there was no significant difference in anaemia and Hb level at 12-months compared to the baseline data.
Variables
Bivariate analysis
Multivariate analysis
Coefficient
std Err
P-value
Coefficient
std Err
P-value
Gender
0
0.2
0.81
-
-
-
Age (years)
0.3
0.2
0.04*
0.2
0.1
0.15
Helminth infections at baseline
0.1
0.2
0.39
0
0.2
0.74
Helminth infections at 12 months
0.6
0.3
0.08
-
-
-
Malaria infection at baseline
0.8
0.2
<0.001*
0.8
0.2
<0.001*
Malaria infection at 12 months
-0.3
0.2
0.1
-
-
-
Antimalarial intervention regim
SP
0
0.2
0.78
0.1
0.2
0.73
SP/PQ
0.4
0.2
0.02*
0.5
0.2
0.01*
*Significant at p<0.05.
Table 3: Multivariate analysis of the effect of anthelminthic treatment on Hb level controlling for antimalarial intervention in schoolchildren in Kinshasa.
Discussion
In this study, we evaluated the impact of anthelminthic treatment using PZQ and ALB, given as non-investigational drugs, on helminth infections, anaemia, and Hb level in the context of an malaria RCT enrolling asymptomatic schoolchildren to evaluate the efficacy and safety of IPTsc with SP or SP+PQ on anaemia and malaria morbidity in Congolese schoolchildren.
Our findings showed that anthelminthic treatment (ALB + PZQ) sustainably reduced the prevalence of helminth infections among schoolchilden by at least 80%. The impact of anthelminthic drugs on anaemia and Hb level on top of the increase due to malaria drugs was not proven in this study. All schoolchildren received the anthelminthic drugs, and the only significant changes were observed in the SP+PQ group (p=0.01) and malaria infection (p< 0.001). Since the present study was not primarily developed to evaluate the effectiveness of anthelmintic treatment, had at least the merit to provide additional evidence of the positive impact of the administration of anthelmintic on the infection in schoolchildren.
The efficacy demonstrated by this therapeutic scheme makes it a promising approach for the control of helminth infections (SCH and STH) in schoolchildren. Indeed, for WHO, anthelminthic treatment using ALB and PZQ remains molecules of choice to fight against helminths [26-28]. These two molecules have been adopted regarding their effectiveness and tolerability. Their impact on the prevalence and intensity of infection and their contribution to improving Hb level in schoolchildren have been reported in several studies [26,29].
Similar to our findings concerning helminth infections, anthelminthic treatment impacted positively the prevalence and intensity of helminth infections among schoolchildren in North- West Ethiopia [29] and Central Province Kenya [30]. Although treatment significantly reduced the infections in this study from 39.2% at baseline to 7.2% (p<0.001) at 12-month, despite this important reduction, we observed that 6.4% of schoolchildren who were not infected at the baseline time became infected, and 8.5% who were initially infected remained infected 12-months after treatment. This could be explained by the level of transmission rate in the study area what could be high.
Regarding the improvement of the Hb level in our study, we found no evidence of the effect of anthelminthic treatment on improving the Hb level among the schoolchildren, since we found no significant changes in the non-malaria intervention group (Table 2). It is likely that, the lack of the impact of anthelminthic drug found on Hb level and anaemia seen in this study due to the lack of the association found between anaemia and helminth infections at baseline. In fact, anaemia was significantly associated with malaria infection at the baseline and before anthelminthic drug administration. That supports the significant association seen between the increasing mean Hb level with malaria infection (p<0.001) and the use of SP+PQ (p=0.01) in the present study (Table 3). There is an agreement with that reported elsewhere [10,31]. Hürlimann et al. in eastern Côte d’Ivoire [10] found the same results among schoolchildren. In their study, anthelminthic treatment did not have any effect on the Hb level. However, in the same study, the Hb level increases significantly over time among children who were found with the high Plasmodium parasitemia at baseline, and no significant relationship was shown with helminth infection at the baseline. The authors mentioned that their observations were likely due to malaria treatment. The absence of effect of anthelminthic drugs was confirmed in the recent systematic review with meta-analysis performed on malaria and helminth coinfections in children living in endemic countries [31].
Nevertheless, we assume that antimalarial treatment in the IPTsc intervention, especially with the SP+PQ combination (Table 2 and 3), was the best in improving Hb levels and reducing anaemia in our study area.
Conclusion
Given these results, it appears that the administration of anthelminthic treatment would be adequate to substantially reduce the prevalence of helminth infections in schoolchildren but not to rapidly impact on the Hb level.
References
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2019. Weekly Epidemiological Record. 2020; 95: 629-640.
- World Health Organization. Global Health Estimates 2020: Disease burden by Cause, Age, Sex, by Country and by Region, 2000-2019. Geneva, WHO. 2020.
- Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009; 3: e412.
- King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010; 113: 95-104.
- Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers-a review. Int J Parasitol. 2010; 40: 1137-1144.
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soiltransmitted helminth infections: updating the global picture. Trends Parasitol. 2003; 19: 547-551.
- Bonnard P, Kalach N, Cadranel JF, Remoue F, Riveau G, Capron A. Digestive and hepatic signs of schistosomiasis. Gastroenterol Clin Biol. 2000; 24: 409-419.
- Guan M, Han B. Association between intestinal worm infection and malnutrition among rural children aged 9-11 years old in Guizhou Province, China. BMC Public Health. 2019; 19: 1204.
- Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, et al. Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anaemia, and prospects for combining control. Am J Trop Med Hyg. 2007; 77: 88-98.
- Hurlimann E, Houngbedji CA, N’Dri PB, Banninger D, Coulibaly JT, Yap P, et al. Effect of deworming on school-aged children’s physical fitness, cognition and clinical parameters in a malaria-helminth co-endemic area of Cote d’Ivoire. BMC Infect Dis. 2014; 14: 411.
- Gabrielli AF, Montresor A, Chitsulo L, Engels D, Savioli L. Preventive chemotherapy in human helminthiasis: theoretical and operational aspects. Trans R Soc Trop Med Hyg. 2011; 105: 683-693.
- Kaatano GM, Siza JE, Mwanga JR, Min DY, Yong TS, Chai JY, et al. Integrated Schistosomiasis and Soil-Transmitted Helminthiasis Control over Five Years on Kome Island, Tanzania. Korean J Parasitol. 2015; 53: 535-543.
- Ortu G, Assoum M, Wittmann U, Knowles S, Clements M, Ndayishimiye O, et al. The impact of an 8-year mass drug administration programme on prevalence, intensity and co-infections of soil-transmitted helminthiases in Burundi. Parasit Vectors. 2016; 9: 513.
- Madinga J, Linsuke S, Mpabanzi L, Meurs L, Kanobana K, Speybroeck N, et al. Schistosomiasis in the Democratic Republic of Congo: a literature review. Parasit Vectors. 2015; 8: 601.
- Khonde KR, Mbanzulu MK, Bin L. Prevalence of Schistosoma mansoni Infection in Four Health Areas of Kisantu Health Zone, Democratic Republic of the Congo. Adv Med. 2016; 2016: 6596095.
- Linsuke S, Nundu S, Mupoyi S, Mukele R, Mukunda F, Kabongo MM, et al. High prevalence of Schistosoma mansoni in six health areas of - Kasansa health zone, Democratic Republic of the Congo: short report. PLoS Negl Trop Dis. 2014; 8: e3387.
- Nundu SS, Aloni MN, Linsuke SW, Ekila MB, Situakibanza HT, Polman K, et al. Prevalence of geohelminth infections in children living in Kinshasa. Arch Pediatr. 2014; 21: 579-583.
- Matangila JR, Doua JY, Linsuke S, Madinga J, Inocencio da LR, Van Geertruyden JP, et al. Malaria, schistosomiasis and soil transmitted helminth burden and their correlation with anaemia in children attending primary schools in Kinshasa, Democratic Republic of Congo. PLoS One. 2014; 9: e110789.
- Doua JY, Matangila JR, Lutumba P, Van Geertruyden JP. Intermittent preventive treatment: efficacy and safety of sulfadoxine-pyrimethamine and sulfadoxine-pyrimethamine plus piperaquine regimens in schoolchildren of the Democratic Republic of Congo: a study protocol for a randomized controlled trial. Trials. 2013; 24: 14-311.
- Matangila JR, Doua JY, Mitashi P, da Luz RI, Lutumba P, Van Geertruyden JP. Efficacy and safety of intermittent preventive treatment in schoolchildren with sulfadoxine/pyrimethamine (SP) and SP plus piperaquine in Democratic Republic of the Congo: a randomised controlled trial. Int J Antimicrob Agents. 2017; 49: 339-347.
- Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thicksmear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972; 14: 397-400.
- World Health Organization. Helminth control in school-age children: a guide for managers of control programmes, 2nd ed. World Health Organization. Geneva, Switzerland: WHO. 2011.
- Braun-Munzinger RA, Southgate BA. Egg viability in urinary schistosomiasis. I. New methods compared with available methods. J Trop Med Hyg. 1993; 96: 22-27.
- Sanchis-Gomar F, Cortell-Ballester J, Pareja-Galeano H, Banfi G, Lippi G. Hemoglobin point-of-care testing: the HemoCue system. J Lab Autom. 2013; 18: 198-205.
- World Health Organization. Worldwide prevalence of anaemia 1993-2005. WHO Global Database on Anaemia. Geneva, Switzerland: WHO. 2008.
- World Health Organization. Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. Geneva, Switzerland: WHO. 2013.
- Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008; 21: 659-667.
- Danso-Appiah A, Olliaro PL, Donegan S, Sinclair D, Utzinger J. Drugs for treating Schistosoma mansoni infection. Cochrane Database Syst Rev. 2013: CD000528.
- Yimam Y, Degarege A, Erko B. Effect of anthelminthic treatment on helminth infection and related anaemia among school-age children in northwestern Ethiopia. BMC Infect. Dis. 2016: 613.
- Kihara JH, Muhoho N, Njomo D, Mwobobia IK, Josyline K, Mitsui Y, et al. Drug efficacy of praziquantel and albendazole in school children in Mwea Division, Central Province, Kenya. Acta Trop. 2007; 102: 165-171.
- Afolabi MO, Ale BM, Dabira ED, Agbla SC, Bustinduy AL, Ndiaye JLA, et al. Malaria and helminth co-infections in children living in endemic countries: A systematic review with meta-analysis. PLoS Negl Trop Dis. 2021; 15: e0009138.