Case Report
Austin J Pulm Respir Med 2014;1(3): 1011.
A Case of Iliac Compression Syndrome Successfully Treated with Catheter-Directed Thrombolysis: Application of Diagnostic MRI Methods for Phase–Assessment of Venous Thromboses
Takuya Aoki1*, Haruna Hirakawa2, Tetsuya Urano1, Tadashi Abe1 and Jun Koizumi3
1Department of Internal Medicine, Respiratory Division, Tokai University School of Medicine, Japan
2Department of Emergency and Critical Care Center, Tokai University School of Medicine, Japan
3Department of Diagnostic Radiology, Tokai University School of Medicine, Japan
*Corresponding author: Takuya Aoki, Department of Internal Medicine, Respiratory Division, Tokai University School of Medicine,143 Shimokasuya, Isehara, Kanagawa 259-1193, Japan
Received: January 27, 2014; Accepted: May 12, 2014; Published: May 14, 2014
Abstract
A 20–year–old male with pulmonary embolism and deep venous thrombosis due to iliac compression syndrome was successfully treated with anticoagulation and catheter–directed thrombolysis (CDT). We applied a diagnostic magnetic resonance imaging method for thrombus–age assessment. In the acute phase, black blood T2–weighted (BBT2W) imaging showed very low signal intensity (SI) of the venous thrombus. However, in the subacute phase, SI was extremely high, and two months after the onset (chronic phase), BBT2W imaging showed medium SI. Thrombus–age information is essential for successful CDT. Our observations allow clinicians to distinguish between acute, subacute and chronic thromboses, allowing optimal application of CDT.
Keywords: Deep venous thrombosis; Catheter–directed thrombolysis; Magnetic resonance imaging
Introduction
The management of patients with pulmonary embolism (PE) and acute deep venous thrombosis (DVT) is based on international guidelines such as the American College of Chest Physicians’ (ACCP) Evidence–Based Clinical Practice Guidelines and the European Society of Cardiology Guidelines. Anticoagulation and compression therapy are essential elements of post–thrombotic syndrome (PTS) prevention, but are not sufficient to prevent PTS in many DVT patients. The CaVenT study [1], a randomized controlled trial, revealed significant eduction in PTS with the addition of catheter–directed thrombolysis (CDT) to conventional treatment. The ATTRACT study [2], also assessing the efficacy and safety of CDT, is now ongoing. When applying CDT and determining the optimal treatment strategies for proximal DVT, thrombus–age assessment is required. Patients with acute iliofemoral DVT are at high risk for developing recurrent venous thromboembolism and the post–thrombotic syndrome. Prevention of PE is urgent and CDT might be more applicable in the acute phase of DVT. As a subacute or chronic–phase thrombus becomes organized, it becomes increasingly difficult to perform CDT, especially in the chronic phase. Discrimination of the acute from the subacute stage is especially important. However, to date, it has not been possible to determine thrombus–age. Veno–occlusive diseases are suggested to have a spectrum of anatomic patterns [3]. In the chronic form, diffuse vein scarring occurs and frequently distorts and obliterates the normal anatomy. Fresh and organized thrombus is invariably present. This pattern is usually difficult to treat, because acute thrombosis is not the sole pathophysiological mechanism [3].
Case Presentation
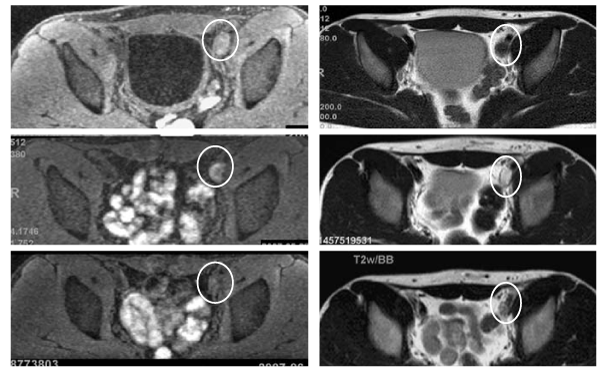
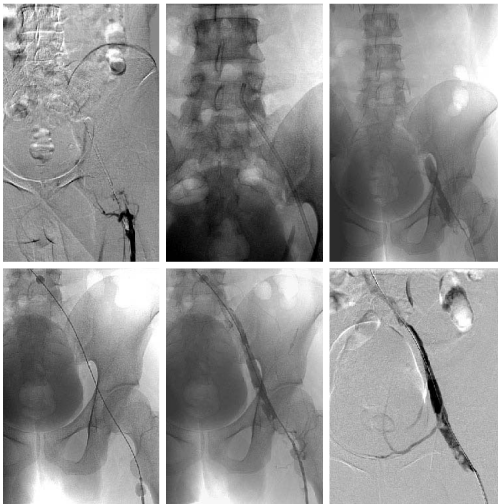
A 20–year–old male consulted our hospital with complaints of and there was no jugular vein distension. On echocardiography, right ventricular size was normal. Thrombopenia (36,000⁄µl) and an increased D–dimer level (104.6 µg⁄ml) were noted. Cardiac troponins were normal. Echography of the lower limb veins revealed a thrombus involving the center of the femoral vein and extending to the periphery of the lower limb veins on the right side, as well as thrombi from the common iliac to the common femoral veins and from the femoral vein to the periphery of the lower limb veins on the left side. Protein C⁄S and AT–III activities were normal. The patient was negative for anti–nuclear antibody (Ab), anti–DNA Ab, lupus anticoagulant, andanti–cardiolipin Ab. One week after the onset (acute phase), direct thrombus (DT) and black blood T2–weighted (BBT2W) magnetic resonance imaging revealed a thrombus extending from the left iliac vein to the IVC. We measured signal intensities (SI) of the thrombus and the iliac bone marrow, and calculated signal intensity ratios (SIR) of the thrombus compared to the adjacent iliac bone marrow. The DT imaging showed a slightly high SIR (1.47; the thrombus SI 1010, the iliac bone marrow SI 689) (Figure 1; the left of the top panels), while the SIR was low (0.357; the thrombus SI 179, the iliac bonemarrow SI 502) on BBT2Wimaging (Figure 1; the right of the top panels). On the same day, computed tomography of pulmonaryarteriography and venography (CTPAV) revealed thrombi in the bilateral pulmonary arteries. Floating thrombi were observed in deep veins of the lower extremities. A retrievable IVC filter was inserted. Anticoagulant therapy was started. Three weeks after the onset (subacute phase), the PE and DVT areas were reduced on CTPAV. Furthermore, the thrombi had been captured by the IVC filter. Onthe same day, BBT2W imaging showed the SIR of the left iliac venous thrombus to be markedly increased (2.72; the thrombus SI 1445, the iliac bone marrow SI 531) (Figure 1; the right of the middle panels), indicating that the nature of the clot had changed as compared to that of the acute phase. Simultaneously, rheolytic thromboaspirationand simple aspiration with a catheter were performed via a left popliteal venous approach (Figure 2; the left and the middle of the upper panels). The aspirated thrombus volume was very small and re–patency was not achieved (Figure 2; the right of the upper panels). Therefore, double–balloon occlusion (Figure 2; the left of the lower panels) and localized high–concentration thrombolytic therapy with an indwelling catheter were conducted (Figure 2; the middle of the lower panels). Despite the right common iliac artery compressing the left common iliac vein, re–patency was achieved (Figure 2; the right of the lower panels). The IVC filter was then removed. The patient was discharged, with maintenance anticoagulant therapy. Two months after the onset (chronic phase), both DT and BBT2W imaging showed approximately iso SIR (DT imaging SIR 1.32; the thrombus SI 794, the iliac bone marrow SI 602, BBT2W imaging SIR 1.14; the thrombus SI 649, the iliac bone marrow SI 568) of the same shrinking left iliac venous thrombus (Figure 1; the left and the right of the bottom panels, respectively). Subsequently, we confirmed complete patency of the bilateral proximal veins after one year.
Figure 1: Direct thrombus (DT) and black blood T2–weighted (BBT2W) magnetic resonance imaging of the left iliac vein thrombus in acute⁄subacute⁄ chronic phases. Serial changes in the signal intensity (SI) of the same thrombus are shown (inside white circles). The top panels show the acute phase, the middle panels the subacute phase, and the bottom panels the chronic phase. The thrombus DT images are on the left side and BBT2W images on the right side. The SI of the thrombus and the adjacent bone marrow were measured, and the signal intensity ratio (SIR) of the thrombus versus the adjacent iliac bone marrow was calculated. In the acute phase, thrombus SIR is rather high (1.47) on DT imaging (top, left panel) while being very low (0.357) on BBT2W imaging (top, right panel). However, BBT2W imaging shows very high SIR (2.72) in the subacute phase thrombus (middle, right panel), strikingly different from that of the acute phase thrombus on BBT2W imaging (top, right panel). The SIR of the same thrombus is isointense on both DT and BBT2W imaging (1.32 and 1.14, respectively) in the chronic phase (bottom, left and right panels, respectively).
Figure 2: Two techniques of catheter-directed thrombolysis: the rheolytic simple aspiration and the double–balloon occlusion technique. The upper panels show angiograms of the conventional rheolytic simple thromboaspiration, and the lower panels show the double–balloon occlusion technique. The catheter was inserted via a left popliteal venous approach (left, upper panel). Rheolytic simple aspiration was performed (middle, upper panel), while the aspirated thrombus volume was very small, and failed to achieve re–patency (right, upper panel). Double–balloon occlusion (left, lower panel) and thrombolytic therapy with an indwelling catheter were then performed (middle, lower panel). The double–balloon occlusion of the vein made it possible to administer topical high–concentration thrombolytic agents via the indwelling catheter without systemic effects. The dissolved clots were aspirated employing the same indwelling catheter. Although thrombi persisted, re–patency was achieved (right, lower panel).
Discussion
The present report provides the first visible evidence showing that phase assessment of venous thrombi is possible by using DT⁄ BBT2W imaging techniques. Venous thrombi, which have low SIR on BBT2W and high SIR on DT imaging, are indicated to be in the acute phase. Thus, prevention of thromboembolism is urgent. On the other hand, high or iso SIR on BBT2W imaging indicate a subacute or chronic phase, respectively. Direct thrombus imaging, which facilitates direct detection of thrombi, was initially reported by Moody [4] in 1997. This technology has since been applied to the diagnosis of several thrombotic disorders. Previous studies employed DT MRI to detect lower limb deep–vein thrombosis [5–7], ischemic heart disease involving the coronary arteries (using a contrast agent) [8,9] and upper thoracic aortic and arch plaques in the presence of cerebrovascular disease [10]. Westerbeek [7] reported the sensitivity and specificity of DT imaging in patients with acute DVT of the lower limbs to be 95 and 100%, respectively. In addition, they indicated normalization on follow–up DT imaging after 3 and 6 months. However, these observations mean that high SI on DT imaging persists from the acute through the subacute or even chronic phase. Herein, we investigated serial changes in thrombi using BBT2Wimaging in addition to DT imaging in a patient with PE⁄DVT related to iliac compression. Three weeks after the onset, there were changes in the SI and SIR which were especially evident onBBT2W imaging, suggesting the subacute phase thrombus to be different from that in the acute phase. These phenomena mean that an earlier change from low to high SIR was detectable on BBT2W imaging but not on DT imaging. Limitations as a single case report include that there is no validation of the accuracy of the observed SIR, and that there is no way to determine the range of SIR that might characterize the age of a clot, such that further prospective investigations are necessary.
For fresh clots, rheolytic thromboaspiration or simple aspiration is widely adopted because it can be quickly performed without the bleeding risk associated with thrombolytic drugs. However, in the present case, the aspirated thrombus volume was very small with rheolytic thromboaspiration, suggesting that thrombi in the subacute phase are not readily released from the vascular wall. Therefore, with a subacute thrombus demonstrating very high SI and SIR on BBT2W imaging, removal is very difficult using conventional CDT. Local thrombolysis under the double–balloon occlusion method is more effective than thromboaspiration, although there is a possibility that the first rheolytic thromboaspiration facilitated the subsequent procedure. With this treatment strategy, high–concentration thrombolytic agents can be topically administered without causing systemic side effects.
A second occurrence of pulmonary embolism might have been fatal in this patient. We thus initially inserted an IVC filter before CDT, because floating thrombi were observed and CDT might have induced new floating thrombi. Indeed, many thrombi were captured inside the IVC filter, as demonstrated upon removal of the filter. Because this patient was quite young and a complete cure was possible, a retrievable filter was inserted. The re–patency rate is reportedly low in patients with iliac compression syndrome [11]. In our patient, restoration of full patency was confirmed during subsequent followup without insertion of a stent. Organized thrombus–related venous stenosis and occlusion may lead to the appearance of a new thrombus. Complete thrombus removal in the subacute phase may thus have been effective in restoring patency in our present patient.
Conclusion
By using BBT2W imaging in addition to DT imaging, we succeeded in demonstrating sequential thrombus signal changes from the acute through chronic phases in a patient with PE and DVT due to iliac compression syndrome. This patient was successfully treated with CDT. Our observations raise the possibility of DT⁄BBT2W imaging becoming an important strategy for both diagnosis and treatment of PE⁄DVT, because thrombus age can be precisely estimated based not only on the presence of a thrombus but also its SIR.
References
- Enden T, Haig Y, Klow NE, Slagsvold CE, Sandvik L, Ghanima W, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomized controlled trial. Lancet. 2012; 379: 31-38.
- Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT Study: A multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J 2013; 165: 523-530.
- Sharii M, Mehdipour M. Percutaneous therapy of acute on chronic lower extremity venous occlusive disease. Catheter Cardiovasc Interv. 2010; 75: 685-689.
- Moody AR. Direct imaging of deep-vein thrombosis with magnetic resonance imaging. Lancet. 1997; 350: 1073.
- Koizumi J, Horie T, Muro I, Kimura E, Shimizu K, Orii M, et al. Magnetic resonance venography of the lower limb. Int Angiol. 2007; 26: 171-182.
- Schmitz SA, O’Regan DP, Gibson D, Cunningham C, Fitzpatrick J, Allsop J, et al. Technical report: Magnetic resonance direct thrombus imaging at 3 T ield strength in patients with lower limb deep vein thrombosis: a feasibility study. Clin Radiol. 2006; 61: 282-286.
- Westerbeek RE, Van Rooden CJ, Tan M, Van Gils AP, Kok S, De Bats MJ, et al. Magnetic resonance direct thrombus imaging of the evolution of acute deep vein thrombosis of the leg. J Thromb Haemost. 2008; 6: 1087-1092.
- Spuentrup E, Botnar RM. Coronary magnetic resonance imaging: visualization of the vessel lumen and the vessel wall and molecular imaging of arteriothrombosis. Eur Radiol. 2006; 16: 1-14.
- Wilensky RL, Song HK, Ferrari VA. Role of magnetic resonance and intravascular magnetic resonance in the detection of vulnerable plaques. J Am Coll Cardiol. 2006; 47: C48-56.
- Bitar R, Moody AR, Leung G, Kiss A, Gladstone D, Sahlas DJ, et al. In vivo identiication of complicated upper thoracic aorta and arch vessel plaque by MR direct thrombus imaging in patients investigated for cerebrovascular disease. AJR Am J Roentgenol. 2006; 187: 228-234.
- Fraser DG, Moody AR, Morgan PS, Martel A. Iliac compression syndrome and recanalization of femoropopliteal and iliac venous thrombosis: a prospective study with magnetic resonance venography. J Vasc Surg. 2004; 40: 612-619.