Case Report
Austin J Pulm Respir Med 2014;1(4): 1018.
Probe-based Confocal Laser Endomicroscopy (pCLE) in the Diagnosis of Diffuse Parenchymal Lung Diseases: Two Cases
Levent Dalar1*, Cengiz Özdemir2, Sinem Nedime Sökücü2, Ahmet Levent Karasulu2, Sedat Altin2, Neslihan Fener3 and Filiz Kosar2
1Department of Pulmonary Medicine, Istanbul Bilim University, Turkey
2Department of Pulmonology, Yedikule Teaching Hospital for Pulmonology and Thoracic Surgery, Turkey
3Department of Pathology, Yedikule Teaching Hospital for Pulmonology and Thoracic Surgery, Turkey
*Corresponding author: Dalar L, Department of Pulmonary Medicine, School of Medicine, Istanbul Bilim University, Sisli Florence Nightingale Hospital, Abidei Hurriyet cad. No: 164, 34370 Sisli, Istanbul, Turkey
Received: June 26, 2014; Accepted: August 25, 2014; Published: August 28, 2014
Abstract
Probe-based confocal laser endomicroscopy (pCLE) has been used in bronchology since 2005. It is a promising method, especially in the diagnosis of parenchymal lung diseases, peripheral nodules, and post-lung transplantation rejection. Optical biopsy samples from pCLE have been previously published and discussed in the diagnosis of some parenchymal diseases. The present article discussed the optical biopsy imaging of a case with Pneumocystis jirovecii pneumonia and a case with early pulmonary fibrosis, which were obtained via pCLE. These are the first two cases in which this method has been used.
Keywords: Interstitial; Optical biopsy; Pneumocystis pneumonia; Pulmonary fibrosis
Introduction
The working principle of confocal endomicroscopy was first described in 1957 and it is based on the narrow-point illumination of the light source focused on a single point in the sample and the return of the reflected light through a small opening or a pinhole in the probe pathway [1-3]. This procedure allows for the exclusion of the information from outside, under, and above the focused area, and to obtain high quality images of a very thin focusing field. It is called “confocal” due to having both illumination and detection systems in the same focal plane. In this way, since there is no longer a need to obtain a biopsy from the targeted area or at least due to the biopsies that can be taken from the exactly targeted area and from the spot, a diagnosis can be achieved.
CellVizio (Mauna Kea Tech, Paris, France) confocal endomicroscopy system employs a proximal scanning system. Confocal microscopy allows in vivo imaging of cells and tissues by fragmentation through obtaining lateral and axial resolutions.
When stimulated by a laser light of 488 nm, various extracellular and cellular fluorophores containing intracellular flavines resulting from the epithelial cells and the cross links between the collagen and elastin in the subepithelial area are resolved at different concentrations.
The microspectrometer studies demonstrated that the main fluorescence signal is spread by the elastin component of human bronchial and alveolar system at this wavelength. It can be easily performed during a routine fiber optic bronchoscopy applied under local anesthesia [4,5]. The in vivo bronchial pCLE imaging technique is simple: a mini-probe is placed through the 2-mm operating channel of the bronchoscope and the probe tip is applied over the bronchial mucosa under visual inspection. With a 50 μm under contact surface for the depth of the focus, the system may likely provide imaging of the basal lamina densa and lamina reticularis, the first layers of the bronchial subepithelial connective tissue. Elastin is found in the acinus, axial backbone of the alveolar ducts, and alveolar entries, and also in the outer sheath of the extra alveolar microvessels. Alveolar fluorescent imaging in active smokers severely differs from the imaging performed in non-smokers. The alveolar areas of the smokers are generally full of high florescent cells corresponding to the alveolar florescent macrophages [4,6].
In the present article, the potentials of the system were discussed, accompanied by the optical biopsy images of a case with Pneumocystis jirovecii pneumonia (PCP) and a case with early pulmonary fibrosis, of which the imaging of distal airways was performed via probe-based, fibered confocal laser endomicroscopy (pCLE) by using CellVizio (Mauna Kea Technologies, Paris, France).
Cases
Case 1
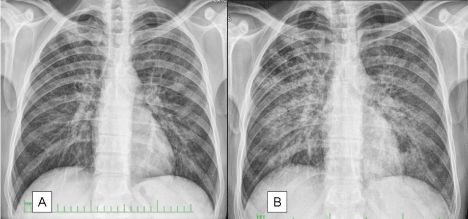
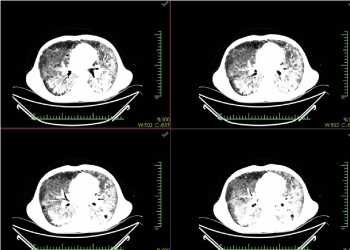
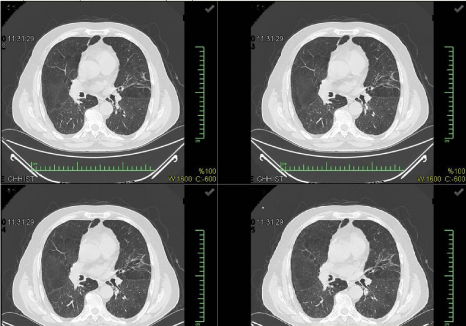
A 32-year-old male patient who admitted to our polyclinic with shortness of breath, cough, sputum, and fever did not have any known medical history and he had shortness of breath, fever, cough, and sputum for two months. He was charged under the pre-diagnosis of pneumonia. The patient was taken into the intensive care unit (ICU) upon the worsening of respiratory failure. The patient was monitored in the ICU via the support of non-invasive mechanical ventilation (NIMV) and the HIV serology was found positive. Since the radiological and clinical appearances were consistent, P. jirovecii pneumonia was considered. Figures 1 & 2 present the chest X-rays and the computed tomography sections of thorax of the patient.
Figure 1: The lung graphies of Case 1 at the first admission and prior to the bronchoscopy. There is significantly increased infiltration.
Figure 2: The images from the computed tomography of thorax of Case 1.
The patient had a history of smoking 30packs/year and he had six months of regular treatment due to tuberculosis in 1992.
Despite the coarse rales heard in the entire bilateral lung areas in the physical examination, tachypnea and distinct oxygen support, no significant characteristics were found other than the oxygen saturation being 85%.
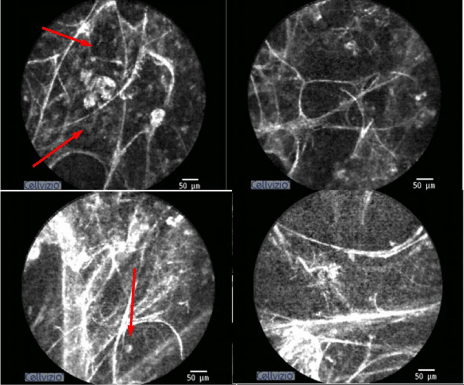
Proper antibiotics, prophylactic treatment, and ventilatory support were administered to the patient. In clinical terms, PCP was considered and a clinical response was achieved to the treatment administered. Before referring for antiretroviral treatment, alveoloscopy was performed for the imaging of the alveolar area and the fluorescent cellular structures within this area (Figure 3). The consolidation and the rich cellular content observed in the patient’s alveolar area was consistent with pneumonia. Consent was obtained to use the procedure findings for scientific purposes.
Figure 3: The parenchyma images of Case 1, obtained via CellVizio (Mauna Kea Tech., Paris, France) probe-based confocal endomicroscopy system. There are fluorescent cellular structures and distinct exudation within the partly-deteriorated alveolar structure.
Case 2
A 63-year-old male patient admitted with cough, shortness of breath, low back pain, and fatigue was diagnosed COAH and initiated with bronchodilator therapy for four months. However, despite regular treatment, his cough did not recover. There were no background and family history. The patient had a history of smoking 20packages/year and he had not smoked for 20 years.
Other than mild desaturation (spO2 93%) observed in room air during the physical examination, end-inspiratory rales were heard in the bilateral basal lung; nothing was found in other system examinations.
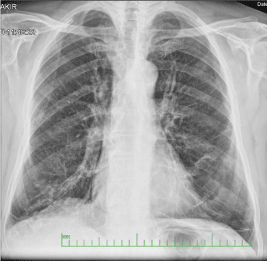
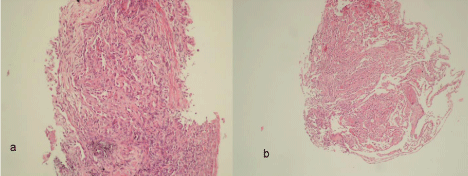
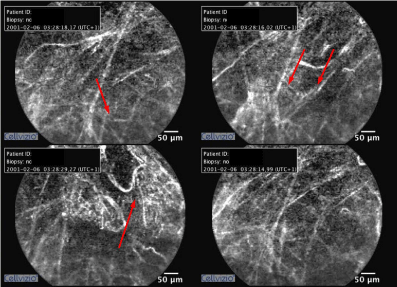
Nothing was identified in the routine blood tests. The chest X-rays and thorax CT sections of the patient, who was considered to have early pulmonary fibrosis with the radiological appearance, are presented in Figures 4 & 5. The result of transbronchial biopsy was consistent with the usual interstitial pneumonia (Figure 6). The imaging of the alveolar structure was performed via confocal endomicroscopy (Figure 7). It was established that the architecture of the elastin structure comprising the alveolar wall was lost and disintegrated, and the alveolar area was significantly poor in cells. Consent was obtained to use the procedure findings for scientific purposes.
Figure 4: There are increased reticular shadows in the lower parts of the Chest X-ray of Case 2.
Figure 5: The images from the computed tomography of thorax of Case 2.
Figure 6: a) Macrophages in alveolary spaces, the proliferation of Type II pneumocytes and increasing of connective tissue in focally (HEX100) B) The lymphoid cells in interstitial area, edema and loose connective tissue proliferation in focal plane (HEX100).
Figure 7: The parenchyma images of Case 2, obtained via CellVizio (Mauna Kea Tech., Paris, France) probe-based confocal endomicroscopy system. The elastin structure of the alveoli is significantly deteriorated and disintegrated.
Discussion
The previous studies have demonstrated that 50% of the peripheral lung support tissues consist of elastin [4]. At the level of the alveoli, elastin comprises the basic structure of alveolar sac and sac entries. Furthermore, the external sheaths of the extra-alveolar microvessels are made of elastin. This basic information allows for the explanation of intra acinar pCLE images. The first in vivo studies with pCLE demonstrated that the procedure was safe and well tolerated in awake individuals under local anesthesia and spontaneous ventilation. Additionally, contrary to the frequently observed during the transbronchial biopsy, acinar imaging does not cause significant bleeding in the proximal airways. This may be explained by the change to the low pressure in the alveolar capillaries while advancing the probe. Moreover, the flat surface design of the probe tip allows the probe to move without damaging the extra-alveolar vessels during shifting. None of the studies conducted to date have reported pleural complications [1,2].
With the pCLE probe safely advanced until the alveolar cavity, it has been possible to obtain information on many parenchymal diseases. Salaün et al. [7] published an alveolar proteinosis case, for which they had an image with pCLE. In the said case, there were diffuse alveolar and interstitial opacities in the lung graphies, and confocal endomicroscopy enabled the imaging of globular lipoproteinaceous material. This finding was confirmed also with the examination of bronchoalveolar lavage.
On the other hand, Finler et al. [8] analyzed the images in different pathological conditions and investigated the repeatability in technical terms. In the said study, the researchers highlighted the fact that the images from pCLE might be reliably evaluated through the thickness and brightness of the fiber, which varied between the different observers, in the different examinations of the same observer and also based on the anatomical location during the re-evaluation of the same patient. They stated that their findings supported the use in the diseases involving respiratory bronchiole. The authors suggested that the normal and pathologically-proven diseased tissues and malignant lesions might be differentiated via this approach. However, they also reported that they had difficulties in differentiating the inflamed lung tissue and malignant lesions via this imaging. They stated that establishing an image library, and analyzing and recording of these images would be a guide for future studies.
Newton et al. [9] analyzed 116 bronchopulmonary segments from 38 patients and four healthy subjects in their study and they demonstrated that the pCLE images were correlated with the results of the high-resolution computed tomography (HRCT) of the thorax and the biopsy. The primary objective of their study was to demonstrate the image differences upon the imaging of the structural modifications occurred in various parenchymal lung diseases via confocal endomicroscopy and to establish the role of pCLE in such diseases. The elastin-rich structure of the lung, as distinct from the gastrointestinal system, requires the use of exogenous fluorescent contrast agent, such as intravenous fluorescein, or topical acriflavine in order to clearly exhibit the boundaries of the cellular architecture and to display the epithelium-embedded gland structures and dynamic in vivo blood flow. The authors reported the secondary objective of the said study was to identify the additional advantages provided (such as differentiating the vessel structures clearly) via in vivo pCLE with the use of fluorescein in the healthy and diseased tissues. Finally, the safety of using pCLE in patients with parenchymal lung diseases was described as the tertiary objective. At the end of the study, the authors stated that they observed some advantages of pCLE compared to the conventional histological examination. They observed both dynamic cells and cyclic movements due to respiration within the acinus. They suggested that the advantages provided by this would be better described by the future studies. They expressed that it was not possible to use the images they obtained to use for final diagnosis at the moment and despite the advantage of observing cellular movement, many data could not be seen and this was a significant disadvantage compared to the conventional histological examination. The mentioned study is the first published study in this field to date, and the optical biopsy image patterns specific to the distinct diseases have not yet been identified. All studies performed using pCLE summarized in Table 1.
Author
Year
Country
n
Study design
Disease
Sensitivity
Specificity
Accuracy
Conclusion
Sorokina et al [10]
2014
Russia
18
Ex vivo
Lung cancer
ns
ns
ns
Succesfull to identify carcinoma
Yserbyt et al [11]
2014
Belgium
105
In vivo
AR in lung Tpx
0.93
0.83
ns
Existence of specific pCLE characteristics in AR
Yserbyt et al [12]
2013
Belgium
26
In vivo
Lung Tpx
ns
ns
ns
Test–retest reliability is good for cellularity and autofluorescence quantification
Yserbyt et al [13]
2013
Belgium
2
In vivo
PAM
ns
ns
ns
describing the endoscopic findings of calcium-phosphate microliths accumulate
Yserbyt et al [14]
2013
Belgium
5
In vivo
Miscellaneous
ns
ns
ns
Needs standardisation of measurement techniques and
evaluation of regional differences in diffuse lung diseases
Morisse et al. [15]
2013
France
13
Animal
Pulmonary aspergillosis
ns
ns
ns
Basis for in vivo diagnosis of aspergillosis
Salaün et al [16]
2013
France
36
In vivo
Amiodarone related pneumonia
1.0
0.88
ns
Succesfull to identify amiodarone related pneumonia
Fuchs et al [17]
2013
Germany
32
In vivo
Lung cancer
0.96
0.87
0.91
May enable the rapid diagnosis of neoplasia during endoscopy
Newton et al [9]
2012
United Kingdom
42
In vivo
DPLD
ns
ns
ns
A basis for future work to harness pCLE
Fuchs et al [18]
2011
Germany
19
In vivo
Miscellaneous
ns
ns
ns
Fluorescein-aided CLE of the lung is safe
Table 1: All studies performed using pCLE summarized.
The present article discussed the imaging of the fluorescent cellular structures within the alveoli, which were full of diffuse exudate in a patient with PCP and also the imaging of the deterioration in the alveolar structure and the alveolar cavity, which was poor in cell in a patient with early IPF. In the first case, a distinct exudation and cellular structures with apparent autofluorescence in the alveolar and acinar area were observed. Although there are no similar published cases yet, a limited number of cases included in the ongoing “Optical Biopsy Lung Atlas” studies exhibit similar findings. It has not yet been possible to identify the disease-specific patterns. On the other hand, the images obtained in the second case are consistent with the study by Newton et al. [9], which is the most extensive study published so far. The deterioration of the alveolar structure and the unaccompanied cellular increase and infiltration are consistent with the images from the optical biopsies of other patients with IPF. Since the purpose of this procedure was the imaging of the specific parenchymal status via pCLE in both cases, the procedure did not result in any changes to the treatment approach. Although the findings specific to the diseases is still at the level of identification, it may be estimated that some various approaches may be produced in the diagnosis and etiology of interstitial lung diseases with the confocal endomicroscopy. For instance, it will be possible over time to observe asbestos fiber directly within the alveolar structure or to analyze and identify various toxic particles upon the development of staining techniques. On the other hand, another purpose for pCLE imaging is to remove the need for transbronchial biopsy during the surveillance bronchoscopy in the lung transplantation cases. The identification of findings specific to the disease may aid in the diagnosis without the necessity of more advanced invasive procedures in many cases with in vivo optical biopsy.
Consequently, the probe-based confocal endomicroscopy may be a leading method for the imaging of peripheral lung nodules, interstitial pathologies, bronchial, and bronchial structure modifications. However, the limitations such as the restricted number of images due to the ability of imaging only the elastin fibers and the lack of taking concurrent tissue samples should be overcome.
Monitoring of post-lung transplantation rejection [11] and assessment of early carcinoma in situ may constitute another area of use.
In conclusion, although the findings with pCLE were helpful in suggesting the diagnosis, it did not show that it could alleviate the need for the tissue biopsy, and the histopathological examination to confirm the diagnosis. We still believe that these cases are worth sharing with the medical community, as publishing this data could stimulate further advancement, and development of the in vivo microscopic cellular exam, hopefully leading to reducing the need for performing transbronchial, and open lung biopsies, and the potential risks involved.
The challenge remains, the selection of the alveolar units to be examined in a patchy heterogeneous disease like IPF.
Further description and correlation of the pCLE findings with histopathological examination of lung biopsies may prove helpful in developing pathognomonic criteria for various lung pathologies, which, when combined with radiographic findings, may suffice for making a diagnosis, reducing the need for invasive biopsy techniques.
Further description and correlation of the pCLE findings with histopathological examination of lung biopsies may prove helpful in developing pathognomonic criteria for various lung pathologies, which, when combined with radiographic findings, may suffice for making a diagnosis, reducing the need for invasive biopsy techniques.
References
- Thiberville L, Salaün M, Bourg-Heckly G. In vivo confocal microendoscopy: from the proximal bronvhus down to the pulmonary acinus. Eur Respir Mon. 2010; 48: 73-79.
- Thiberville L, Salaün M. Diagnostic of lung cancer: confocal bronchoscopy. In: Diaz-Jimenez JP, Rodriguez AN, editors. Interventions in pulmonary medicine. Springer New York. 2013: 221-230.
- Vercauteren T. Image Registration and Mosaicing for Dynamic In Vivo Fibered Confocal Microscopy. PhD Thesis, Paris. 2007.
- Thiberville L, Salaün M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, et al. Human in vivo fluorescence microimaging of the alveolar ducts and sacs during bronchoscopy. Eur Respir J. 2009; 33: 974-985.
- Thiberville L, Moreno-Swirc S, Vercauteren T, Peltier E, Cavé C, Bourg Heckly G, et al. In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. Am J Respir Crit Care Med. 2007; 175: 22-31.
- Bourg Heckly G, Thiberville L, Vever-Bizet C, Vielerobe B. In vivo endoscopic autofluorecence micro-spectro-imaging of bronchi and alveoli. Proc SPIE. 2008; 2008: 6851.
- Salaün M, Roussel F, Hauss PA, Lachkar S, Thiberville L. In vivo imaging of pulmonary alveolar proteinosis using confocal endomicroscopy. Eur Respir J. 2010; 36: 451-453.
- Filner JJ, Bonura EJ, Lau ST, Abounasr KK, Naidich D, Morice RC, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchology Interv Pulmonol. 2011; 18: 23-30.
- Newton RC, Kemp SV, Yang GZ, Elson DS, Darzi A, Shah PL, et al. Imaging parenchymal lung diseases with confocal endomicroscopy. Respir Med. 2012; 106: 127-137.
- Sorokina A, Danilevskaya O, Averyanov A, Zabozlaev F, Sazonov D, Yarmus L. Comparative study of ex vivo probe-based confocal laser endomicroscopy and light microscopy in lung cancer diagnostics. Respirology. 2014; 19: 907-913.
- Yserbyt J, Dooms C, Decramer M, Verleden GM. Acute lung allograft rejection: diagnostic role of probe-based confocal laser endomicroscopy of the respiratory tract. J Heart Lung Transplant. 2014; 33: 492-498.
- Yserbyt J, Dooms C, Decramer M, Verleden GM. Probe-based confocal laser endomicroscopy of the respiratory tract: a data consistency analysis. Respir Med. 2013; 107: 1234-1240.
- Yserbyt J, Alamé T, Dooms C, Ninane V. Pulmonary alveolar microlithiasis and probe-based confocal laser endomicroscopy. J Bronchology Interv Pulmonol. 2013; 20: 159-163.
- Yserbyt J, Dooms C, Ninane V, Decramer M, Verleden G. Perspectives using probe-based confocal laser endomicroscopy of the respiratory tract. Swiss Med Wkly. 2013; 143: w13764.
- Morisse H, Heyman L, Salaün M, Favennec L, Picquenot JM, Bohn P, et al. In vivo molecular microimaging of pulmonary aspergillosis. Med Mycol. 2013; 51: 352-360.
- Salaün M, Roussel F, Bourg-Heckly G, Vever-Bizet C, Dominique S, Genevois A, et al. In vivo probe-based confocal laser endomicroscopy in amiodarone-related pneumonia. Eur Respir J. 2013; 42: 1646-1658.
- Fuchs FS, Zirlik S, Hildner K, Schubert J, Vieth M, Neurath MF. Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur Respir J. 2013; 41: 1401-1408.
- Fuchs FS, Zirlik S, Hildner K, Frieser M, Ganslmayer M, Schwarz S, et al. Fluorescein-aided confocal laser endomicroscopy of the lung. Respiration. 2011; 81: 32-38.