
Review Article
Austin J Pulm Respir Med. 2024; 11(1): 1102.
Non-Invasive Mechanical Ventilation and Mortality as Ventilatory Strategies in The Hospital Environment During Covid-19: A Systematic Review
Beatriz Boaventura de Carvalho Alves¹; Mariana Machado dos Santos¹; Yone Kauane da Silva Lima²; Maria Cecília Pires dos Santos²; Laisa Liane Paineiras-Domingos³*
¹Physiotherapist, Multidisciplinary Institute of Rehabilitation and Health, Federal University of Bahia, Brazil
²Graduating in Physiotherapy, Multidisciplinary Institute of Rehabilitation and Health, Federal University of Bahia. Padre Feijó, Brazil
³Adjunct Professor, Department of Physical Therapy, Multidisciplinary Institute of Rehabilitation and Health, Federal University of Bahia, Salvador (BA), Brazil
*Corresponding author: Laisa Liane Paineiras-Domingos PhD, Adjunct professor, Department of Physical Therapy, Multidisciplinary Institute of Rehabilitation and Health, Federal University of Bahia, Salvador, Bahia, Brazil. Tel: +5571 996664372 Email: laisanit@gmail.com
Received: May 24, 2024 Accepted: June 12, 2024 Published: June 19, 2024
Abstract
Objective: To synthesize mortality as an outcome of non-invasive ventilation in patients hospitalized for COVID-19 in the hospital environment.
Methods: This is a systematic review that followed the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Checklist (PRISMA), under registration PROSPERO (CRD42022360052). They were included cohort studies available in any language with the participation of individuals hospitalized in hospital units for COVID-19, with the Non-Invasive ventilation (NIV) as the main physical therapy procedure. The outcomes of interest were mortality, type of adaptations, prolongation of life, intubation, dyspnea control, length of stay, length of NIV use, saturation, and eligibility.
Results: Eight articles were included. The most cited NIV modes were the continuous positive airway pressure (CPAP) and the BI-level Positive Airway Pressure (BIPAP), with the Helmet interface being the most used. NIV failure was defined as a determinant for intubation or hospital death. Days with NIV support before ICU admission and age were identified as potential risk factors for higher in-hospital mortality.
Conclusion: Although we understand that data on this strategy are still limited, studies have shown that NIV, even in a non-intensive environment, can be effective for the treatment of SARS-Cov-2, provided there is an attentive and continuous therapeutic approach.
Implications for Clinical Practice: The NIV can be effective for the treatment of SARS-Cov-2 and it has been suggested as the main physiotherapeutic approach for individuals hospitalized due to COVID-19. The NIV can be used to stabilize the clinical course of patients affected by mild/ moderate acute respiratory failure due to COVID -19 and it is associated with an improvement in the PaO2/FiO2 ratio.
Keywords: Non-invasive ventilation; COVID-19; Hospitals; Pulmonary Ventilation; Mortality
Introduction
Covid-19 is an acute respiratory infection caused by the SARS-CoV-2 coronavirus, highly transmissible and globally distributed. It usually results in a severe form of viral pneumonia, severe acute respiratory syndrome (SARS) [1]. The rapid and disorderly growth of COVID-19 cases has established a health pandemic in the world, requiring a rapid response to emerging events of the disease in view of the aggressive impacts on the health of affected patients and the facilitated form of transmission. The infection causes major impairments to the respiratory system, especially in the ventilation/perfusion (V/Q) ratio. Patients may present severe hypoxemia (PaO2<60mmHg) with normal presentation of PaCO2 levels and dyspnea, which is not necessarily related only to the presence of hypoxemia [2]. However, some patients present with the so-called happy hypoxia, where even with PaO2 levels <60mmHg or SpO2 = 80%, individuals do not experience respiratory distress or do not report difficulty breathing [3].
SARS can be refractory to oxygen therapy, and in this condition invasive or non-invasive ventilatory support is required. Non-Invasive Ventilation (NIV) consists of the use of ventilatory support that does not resort to invasive methods of the airway and its main objectives are to reduce the work of breathing, promote rest of the respiratory muscles and improve gas exchange. In addition to avoiding Orotracheal Iintubation (OTI), when possible, and consequently minimizing the risks associated with this procedure, such as nosocomial infections and tracheal injury. Furthermore, as it does not require sedation, NIV allows the patient to speak, maintain an effective cough and oral feeding. It is easy to use and handle, which can lead to a decrease in hospitalization time, mortality and a decrease in hospital costs [4].
Even with the benefits of NIV on respiratory signs and symptoms, there is still no consensus on its use in COVID-19, since there may be unavailability of resources, considering the reality of the Brazilian health system, and the increase in risk of spreading the virus. However, NIV can be considered for use, if the patient responds, if the above criteria are respected and without postponing OTI [5].
In this way, the study sought to synthesize mortality as an outcome of NIV in patients hospitalized for COVID-19 in the hospital environment.
Methods
This is a systematic review that followed the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Checklist (PRISMA) [6], registered with PROSPERO (CRD42022360052).
Eligibility Criteria
They were included cohort studies available in any language with the participation of individuals hospitalized for COVID-19 and treated with NIV as the main physiotherapeutic approach.
The outcomes of interest were mortality, type of adaptations, prolongation of life, intubation, dyspnea control, length of stay, duration of NIV use, saturation and eligibility. Studies that were not available at the latest resources and that did not address mortality as a study variable were excluded.
Search Strategy
The searches were carried out using the acronym PICOS strategy: Patients hospitalized for Covid-19 (Population); NIV (Intervention); no control (Control); Mortality (Primary outcome); Intubation, dyspnea, length of stay, associated conditions and reason for failure (Secondary outcomes) and Cohort (Type of study).
The search strategy was developed and applied in the PubMed, Scielo, Scopus and PEDro databases, with the association of the descriptors: “Non-invasive ventilation”, “COVID-19,” and “Mortality”, through the Boolean operators AND and OR for greater awareness in the search result.
Data extraction (selection and encoding): At first, the titles and abstracts of the studies found through the search strategy were evaluated by two independent researchers, with the full texts of potentially eligible studies being selected and evaluated. A third researcher would be consulted if there was any doubt or disagreement. The following data were extracted: Author/Year, total sample, characteristics of the studied population, exposure, outcomes related to the use of non-invasive ventilation and results.
Risk of bias: The risk of bias of the studies was assessed using the Cochrane Risk Of Bias In Non-randomized Studies - of Exposures tool for assessing the risk of bias (ROBINS-E tool). The ROBINS-E was designed specifically for use in systematic reviews, where the main focus is the analysis of the causal effect estimated by the result found in cohort studies with exposure [7].
Data summary and synthesis measures: The collected data were grouped and synthesized in a table format in Microsoft Excel 2019 software and then grouped in a synthesized way into two tables to carry out a narrative analysis of the data. Table 1 presents the general characterization of the articles including: Author/year of publication, country of study, sample details as well as mean and Standard Deviation (SD) of the age of each group present in the study, exposure, outcomes, results, while the Table 2 provides information on secondary outcomes, results found and conclusion of the studies.
Results
220 articles were identified in the databases used. 4 articles were excluded after initial screening by titles and removal of duplicates. Then, 19 articles were selected for abstract reading, where 11 articles were excluded for not addressing NIV therapeutically and not being cohort studies. After reading them in full, 8 articles were included in the study, according to the selection flowchart (Figure 1).
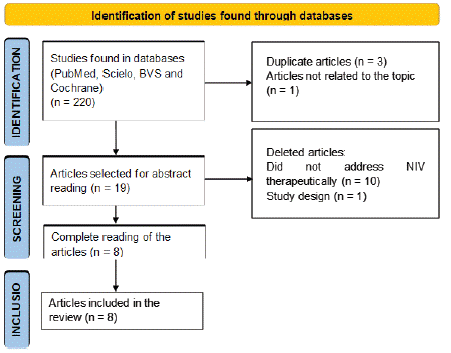
Figure 1: Study search and selection flowchart.
Risk of Bias
Risk of bias analysis judged that 3 studies were “high risk”. The others may provide “some concerns”, according to the detailed analysis (Figure 2). In the graphical representation by domains, it was possible to observe that the greatest risks were identified in the domain of “Selection of reported results” and “Confounding bias” (Figure 3).
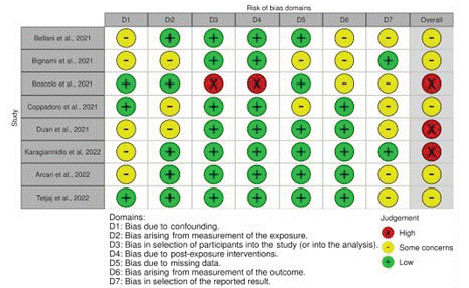
Figure 2: Ranking risk of bias in each domain per study.
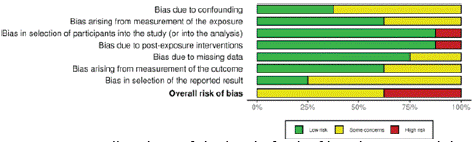
Figure 3: Overall ranking of the level of risk of bias by assessed domain.
General Characteristics of the Studies
We evaluated 8 cohort studies, published between 2021 and 2022, which evaluated the mortality rate and other outcomes in a total sample of 19,728 patients, with a predominance of males. The most cited NIV modes were CPAP [9, 10, 11, 12, 13] and BIPAP [10, 11, 13, 14] with the Helmet interface being the most used.
Table 1 concentrates the main characteristics of the articles included and their main results.
Non-Invasive Ventilation and Mortality: In the observational study by Duan et al. [13] (n=36), which evaluated the use of the High Flow Nasal Cannula (HFNC) and NIV in patients with COVID-19, the results regarding mortality did not show statistically significant differences (4 % vs. 8%, p>0.99). On the other hand, Tetaj et al. [14] analyzed the use of NIV (n=224) and conventional supplemental oxygen therapy (n=718) in 942 patients with Acute Respiratory Distress Syndrome (ARDS) and obtained a mortality rate in the 28 days 96% lower (OR 0.04, 0.01–0.32) in patients who were treated with previous NIV and did not evolve to OTI, than those who followed OTI without having used the resource.
The results of Coppadoro et al. [12] (n=306) with the use of Continuous Positive Airway Pressure (CPAP) with Helmet interface were similar, the strategy was considered viable and effective for use outside the ICU, the mortality of patients in complete treatment was 12.5% (22/176) and all deaths occurred after admission to the ICU, which corroborates the findings of Tetaj et al. [14] who associate mortality with the evolution of the condition to OTI.
Arcari et al. [8] (n=112) observed differences between oxygen therapy using a Venturi mask and NIPPV. In-hospital mortality was 15% (8) in the Venturi mask group and 95% (20) in the NIPPV failure group. In evaluating patients with COVID-19 who received NIV and IMV, Karagiannidis et al. [15] (n=17023) concluded that the mortality rate of patients who successfully received some type of NIV was lower than that of patients who were directly intubated and only received IMV (44% NIV vs. 54% IMV).
NIV-related mortality rates were also compared between patients in and out of the ICU. In the cohort of Bignami et al. [10] (n=231) there was an overall mortality of 56.3% and there were no significant differences between the mortality of patients outside the ICU (57.3%) and those who were admitted to the ICU (54 %). The use of NIV was considered safe and feasible, as it slowed down the simultaneous influx of patients with hypoxemic ARF to intensive care units.
Bellani et al. [9] (n=798) observed differences between NIV failure and success groups, the overall study mortality was 25%. 177 patients died without progressing to OTI 8 (5-13) days after starting NIV. Out of 138 (78%) of these patients, a DNI was performed.
Of the patients treated with NIV outside the ICU in the study by Boscolo et al. [11] (n=280), 54% died, of those treated in the ICU, 36% died, and among those who failed NIV in and out of the ICU, 41% died. The mortality rate was directly and significantly related to NIV duration, increasing in patients who received >2 days compared to those treated for =2 days (63% vs 41%).
Orotracheal intubation: Of the included articles, 5 assessed the outcome of OTI. Duan et al. [13] did not observe differences regarding the rate between the HFNC and NIV groups (17% vs. 15%, p>0.99). However, Arcari et al. [8] found an overall OTI rate of 8% and 42% in the NIPPV failure group.
Tetaj et al. [14], who divided patients into NIV and Non-NIV groups, concluded that patients who required NIV had a greater need for OTI (28.6% vs 5.7%, p<0.001), however, Bellani et al.9 found results that showed that OTI occurred in only 15.4% (123) of the patients, after 5 days of starting NIV. In the study by Karagiannidis et al. [15], a direct relationship between mortality and OTI was observed. Patients successfully treated with NIV had lower rates of OTI than those who went directly to IMV, the same occurred in reverse, those who failed the therapy had higher rates, the difference being more expressive when failure was late.
Reasons associated with NIV failure: The reasons associated with NIV failure in the studies were diverse, including female gender with 2x higher risk than male gender, advanced age (62% for each 10-year increase), hypertension with 2.6x higher risk, diagnosis of Lung Disease Chronic Obstructive Disease (COPD), which confers a 6.2x higher risk and history of neoplasia in the last 5 years with a 3.2x higher risk [14]. In addition to comorbidities and other characteristics that favor structural fragility, respiratory rate (RR) >24ipm was a variable that showed 81% sensitivity and 76% specificity to predict CPAP failure [12].
PaO2/FiO2 ratio: The results regarding the outcome of the PaO2/FiO2 ratio are intertwined. An increase in the variable was observed when analyzing the use of CPAP with Helmet interface, with consequent improvement in oxygenation (100 to 200 mmHg (P <0.001) [12]. In addition, previous values of the outcome were significantly associated with hospital mortality in the study by Boscolo et al. [11]. Bellani et al. [9] found a PaO2/FiO2 ratio associated with NIV failure, which occurred in 18% of patients with a PaO2/FIO2 ratio >150 mmHg and in 53% of patients with a PaO2/FIO2 ratio < 150 mmHg.
Conditions associated with NIV: During the use of the therapy, 9.5% of the patients developed edema of the upper limbs, deep venous thrombosis (DVT) of the upper limbs in 3.5% of the patients and pneumothorax and pleural effusion in 1.7% of them. There were also 3 episodes of nausea or vomiting in patients undergoing NIV (1.3%) and 2 cases of hemoptysis (0.9%) [10].
Dyspnoea: Only one of the studies analyzed the outcome in 631 patients, which had a higher prevalence in the NIV failure group (116 (48.8%)) when compared to the success group (60 (14.5%)) [9].
Discussion
This systematic review highlighted the outcomes related to the use of NIV in patients with COVID-19 and the ventilatory strategies used in the hospital environment in the context of the pandemic. In this period, some studies suggested that NIV would be feasible both inside and outside the ICU [16-18] and, in addition, observational studies have highlighted the importance of the resource in an attempt to stabilize the clinical course of patients affected by mild to moderate acute respiratory failure due to COVID -19 [16,19].
Our findings demonstrated that NIV is associated with an improvement in the PaO2/FiO2 ratio, which, in turn, is directly related to mortality, in addition to being a predictor of NIV failure. Similar results were found by Kaya et al. [20], who analyzed the important prognostic factors in invasive ventilation and NIV in patients with acute hypoxemic respiratory failure resulting from SARS-CoV-2 infection; the authors found a significant difference between the mean of the lowest values, which were lower in the IMV group when compared to the NIV group; in addition, they concluded that patients who started treatment with NIV had relatively low negative prognostic factors and lower mortality.
Another retrospective study that compared NIV with IMV in patients hospitalized with severe pneumonia due to COVID-19 found significant differences between the mortality rates in the groups that underwent invasive ventilation with positive pressure and those that used NIV followed by IMV when compared with patients who used only non-invasive ventilatory support. In addition to higher rates, patients who used invasive ventilation, either alone or after NIV use, also had a higher chance of mortality. The authors concluded that in a resource-limited setting, ventilatory support through NIV is associated with longer survival in these patients; individuals who were intubated early or after trying NIV, in turn, have the same prognosis, with a higher probability of mortality [21].
Arabi et al. [22] evaluated the long-term outcomes of patients with COVID-19 and compared the use of NIV through the Helmet with usual respiratory support and found no significant differences in terms of mortality at 180 days between both groups. However, the authors observed that, despite not being associated with higher mortality, IMV was an independent predictor of lower health-related quality of life.
Regarding orotracheal intubation rates, NIV can reduce the need for IMV when successfully performed, despite being associated with negative outcomes when administered for a prolonged period. An observational study that compared the management and results of the approaches adopted at the beginning of the pandemic and 1 year after this period found similar results. In this study, it was observed that, at the beginning of the pandemic, most patients received invasive ventilatory support, contrary to what was observed in the following year, when more evidence of safety related to NIV increased and its use increased. In this context, it was not possible to establish a direct causal relationship between the decrease in mortality rates and the use of NIV, as this was not the only determining factor. Although, the authors state that NIV seems to be associated with a reduction in the duration of mechanical ventilation and the length of stay in the ICU. However, despite these favorable results, a delay in intubation may be associated with a worse prognosis; however, the mentioned study found no association between a longer delay between admission and the intubation process with the duration of IMV and higher mortality in the group that was intubated late compared to those that were intubated on admission [23].
Kasarabada et al. [24] evaluated the impact of the duration of invasive ventilation strategies and NIV on mortality in patients with COVID-19. They observed that there was a considerable increase in mortality among patients who received IMV after a trial of non-invasive ventilatory support for more than 7 days, regardless of the patients’ previous status related to comorbidity. However, the study demonstrates that age was strongly associated with the mortality outcome, as patients over 65 years of age, when compared to individuals younger than this age, had a higher mortality rate. In line with the present discussion, the authors performed the association between the outcome related to NIV and the main predictors of failure of this approach, advanced age and the presence of comorbidities being the main factors. Unlike our study, however, in which NIV failure was associated with female gender, the authors did not find an association of mortality by gender [24].
With regard to complications and adverse events from NIV, there are few studies that address these issues, with edema in the upper limbs, deep vein thrombosis (DVT), pneumothorax and pleural effusion being the main events cited in the studies analyzed in the present review. Ragnoli et al. [25] evaluated risk factors and outcomes for pneumothorax in patients with severe respiratory failure due to COVID- 19 and concluded that patients who were treated with NIV from admission onwards, when compared to those treated with other ventilatory supports, had a worse prognosis, with a higher risk of developing the complication. In the study of Srinivasaiah et al. [21] in turn, the incidence of pneumothorax was higher in intubated patients. In addition, the authors also observed longer ICU and hospital stays in patients who received IMV since admission when compared to those who used NIV support.
This systematic review presented some limitations. The included studies investigated cohorts carried out during the first wave of the COVID-19 pandemic, in 2020. Thus, it is necessary to consider the critical issues of the period, characterized by the scarcity of information for a well-defined therapeutic approach and the crisis of hospital capacity. Most studies were carried out in Italy, the first western country to suffer from high numbers of contaminations and deaths, in this sense, it is important to understand political differences, clinical practice and sociocultural characteristics. Despite bringing relevant data and demonstrating efficacy in the treatment of SARS-Cov-2, it has not yet been possible to conclude the effectiveness of NIV strategies in reducing mortality.
Conclusion
NIV as a therapeutic strategy for COVID-19 is still a tool that presents challenges in its administration. Despite the concerns and scarcity of information during the pandemic period, it was presented as viable and effective to be used, given the need for ventilatory assistance in these patients. Therefore, our findings need to be confirmed by future investigations that address the same outcomes involving patients from the COVID-19 waves following 2020 as a population.
Highlights
• The NIV can be effective for the treatment of SARS-Cov-2.
• The NIV has been suggested as the main physiotherapeutic approach for individuals hospitalized due to COVID-19.
• The NIV can be used to stabilize the clinical course of patients affected by mild/ moderate acute respiratory failure due to COVID -19.
Author Statements
Declaration of Interest Statement
The authors report that they have no competing interests to declare.
References
- Velavan TP, Meyer CG. The COVID-19 epidemic. Tropical medicine & international health. 2020; 25: 278-280.
- Simonson TS, Baker TL, Banzett RB, Bishop T, Dempsey JA, Feldman JL, et al. Silent hypoxaemia in COVID-19 patients. The Journal of physiology. 2021; 599: 1057-1065.
- Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. American journal of respiratory and critical care medicine. 2020; 202: 356-360.
- Perkins GD, Mistry D, Gates S, Gao F, Snelson C, Hart N, et al. Effect of protocolized weaning with early extubation to noninvasive ventilation vs invasive weaning on time to release from mechanical ventilation among patients with respiratory failure: the breathe randomized clinical trial. Jama. 2018; 320: 1881-1888.
- Martinez BP, de Andrade FMD, Roncalli Â, Martins JA, da Cunha Ribeiro D, Pianezzola E, et al. Indication and use of non-invasive ventilation and high-flow nasal cannula, and guidance on the management of invasive mechanical ventilation in the treatment of acute respiratory failure in COVID-19. Assobrafir Science. 2020; 11: 101-110.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.). 2021; 372: n71.
- ROBINS-E Development Group (Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R, et al). Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E). 2022.
- Arcari L, Ciolina F, Cacciotti L, Danti M, Camastra G, Manzo D, et al. Semiquantitative Chest CT Severity Score Predicts Failure of Noninvasive Positive-Pressure Ventilation in Patients Hospitalized for COVID-19 Pneumonia. Journal of cardiothoracic and vascular anesthesia. 2022; 36: 2278-2286.
- Bellani G, Grasselli G, Cecconi M, Antolini L, Borelli M, De Giacomi F, et al. Noninvasive ventilatory support of patients with COVID-19 outside the intensive care units (WARd-COVID). Annals of the American Thoracic Society. 2021; 18: 1020-1026.
- Bignami E, Bellini V, Maspero G, Pifferi B, Fortunati Rossi L, Ticinesi A, et al. COVID-19 respiratory support outside the ICU’s doors. An observational study for a new operative strategy. Acta Bio Medica: Atenei Parmensis. 2021; 92: e2021365.
- Boscolo A, Pasin L, Sella N, Pretto C, Tocco M, Tamburini E, et al. Outcomes of COVID-19 patients intubated after failure of non-invasive ventilation: a multicenter observational study. Scientific reports. 2021; 11: 17730.
- Coppadoro A, Benini A, Fruscio R, Verga L, Mazzola P, Bellelli G, et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: an observational study during the COVID-19 outbreak. Critical Care. 2021; 25: 80.
- Duan J, Chen B, Liu X, Shu W, Zhao W, Li J, et al. Use of high-flow nasal cannula and noninvasive ventilation in patients with COVID-19: A multicenter observational study. The American Journal of Emergency Medicine. 2021; 46: 276-281.
- Tetaj N, Piselli P, Zito S, De Angelis G, Marini MC, Rubino D, et al. Timing and outcomes of noninvasive ventilation in 307 ARDS COVID-19 patients: an observational study in an Italian third level COVID-19 hospital. Medicine. 2022; 58: 1104.
- Karagiannidis C, Hentschker C, Westhoff M, Weber-Carstens S, Janssens U, Kluge S, et al. Observational study of changes in utilization and outcomes in mechanical ventilation in COVID-19. PloS one. 2022; 17: e0262315.
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323: 1574–81.
- Cammarota G, Esposito T, Azzolina D, Cosentini R, Menzella F, Aliberti S, et al. Noninvasive respiratory support outside the intensive care unit for acute respiratory failure related to coronavirus-19 disease: a systematic review and meta-analysis. Crit Care. 2021;25: 268.
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; 382: 1708–20.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respire Med. 2020; 8: 475–81.
- Kaya Gök A, Turkmen A, Köse E, Çengel F, Sehirlioglu S. Correlation of important prognostic factors and CT scores in invasive and non-invasive ventilation of COVID-19 patients. Ulus Travma Acil Cerrahi Derg. 2023; 29: 163-168.
- Srinivasaiah M, Krishnappa Gowda Varma MM, Nandini MG, Chaitra V, Gulur H, Harshitha V. A Retrospective Analysis of Ventilatory Strategy Comparing Non-invasive Ventilation (NIV) With Invasive Ventilation in Patients Admitted with Severe COVID-19 Pneumonia. Cureus. 2023.
- Arabi Yaseen M, Al-Dorzi HM, Aldekhyl S, Al Qahtani S, Abdukahil SA, Al Qasim E, et al. Long-term outcomes of patients with COVID-19 treated with helmet noninvasive ventilation or usual respiratory support: follow-up study of the Helmet-COVID randomized clinical trial. Intensive Care Medicine. 2023; 49: 302-312.
- Rama-Maceiras P, Sanduende Y, Taboada M, Casero M, Leal S, Pita-Romero R, et al. Critical patients COVID-19 has changed the management and outcomes in the ICU after 1 year of the pandemic? A multicenter, prospective, observational study. Infectious diseases and clinical microbiology (English ed). 2023; 41: 70–8.
- Kasarabada A, Barker K, Ganoe T, Clevenger L, Visco C, Gibson J, et al. How long is too long: A retrospective study evaluating the impact of the duration of noninvasive oxygenation support strategies (high flow nasal cannula & BiPAP) on mortality in invasive mechanically ventilated patients with COVID-19. Cortegiani A, editor. PLOS ONE. 2023; 18: e0281859.
- Ragnoli B, Cena T, Radaeli A, Pochetti P, Conti L, Calareso A, et al. Pneumothorax in hospitalized COVID-19 patients with severe respiratory failure: Risk factors and outcome. Respiratory Medicine. 2023; 211: 107194.