
Research Article
Austin J Pulm Respir Med 2015; 2(2): 1026.
Central versus Peripheral Pulmonary Embolism: Analysis of the Impact on the Physiological Parameters and Long- Term Survival
Alonso-Martínez JL*, Anniccherico-Sánchez FJ, Urbieta-Echezarreta M, Villar-García I and RojoÁlvaro J
Department of Internal Medicine, Hospital Complex of Navarra, Spain
*Corresponding author: Alonso-Martínez JL, Department of Internal Medicine, Hospital Complex of Navarra, Irunlarrea 6, 31008 Pamplona, Navarra, Spain
Received: August 05, 2015; Accepted: September 01, 2015; Published: September 03, 2015
Abstract
Background: Studies aimed at assessing whether the emboli lodged in the central pulmonary arteries carry a worse prognosis than more peripheral emboli have yielded controversial results.
Patients and Methods: Consecutive patients diagnosed with acute symptomatic Pulmonary Embolism (PE) by means of computed tomography angiography were evaluated at episode index and traced through the computed system of clinical recording and following-up. Central PE was diagnosed when thrombi were seen in the trunk or in the main pulmonary arteries and peripheral PE when segmental or sub segmental arteries were affected.
Results: A total of 530 consecutive patients diagnosed with pulmonary embolism were evaluated, 255 patients had central PE and 275 patients had segmental or sub segmental PE. Patients with central PE were older, had higher plasma levels of NT-ProBNP, Troponin I, D-dimer, alveolar-arterial gradient and shock index (p<.001 respectively for each one).
Patients with central PE had an all-cause mortality of 40%, while patients with segmental or sub segmental PE had an overall mortality of 27%, OR 1.81 (CI 95% 1.16-1.9).
Survival was lower in patients with central PE than in patients with segmental or sub segmental PE, even after to avoid confounders (p=.018).
Conclusion: Besides greater impact on hemodynamics, gas exchange and right ventricular dysfunction, central pulmonary embolism associates a shorter survival and an increased long-term mortality.
Keywords: Venous thromboembolic disease, Pulmonary embolism, Central pulmonary embolism, Survival, Cardiac peptides
Introduction
With the increasing use of the Computed Tomography (CT) angiography as the main diagnostic method in pulmonary thromboembolism, new approaches for categorizing the severity of pulmonary embolism have been conducted mainly based on thrombus burden and its impact on the right ventricle [1-17].
Data from radiographic studies which used CT angiography to evaluate the prognostic factors associated with pulmonary embolism (such as the relationship between the diameter of the right ventricle and the diameter of the left ventricle, the bowing of the interventricular septum [1-4], the thrombus burden [14-17], the reflux contrast to the cava [18], and the diameter of the pulmonary artery regarding the azygos vein), have been studied as prognostic factors of morbidity and mortality in the context of acute pulmonary thromboembolism.
The impact of pulmonary embolism on the right ventricle measured by biomarkers and D-dimer also has been correlated with the thrombotic burden in several investigations [19,20], and recently the European Society of Cardiology has included the right ventricular dysfunction in the risk assessment of the pulmonary embolism [21] evaluated by echocardiography as well as measured by CT, though in both cases the prediction of an adverse outcome has been difficult to standardize.
The location of the thrombi in the pulmonary arterial tree has received some attention as prognostic factor. Prognosis is worse when the trunk or main pulmonary arteries are occupied by thrombi with either complete or incomplete occlusion [22-25], although this has not been shown consistently in all studies, since several of them have been unable to demonstrate an association between image scores and mortality [26-28].
Although many radiologists consider a pulmonary embolism is massive when thrombi are visualized in the main pulmonary arteries, the current criterion is the state of the blood pressure, categorizing the patients as normotensive or hypotensive patients, with the latter needing fibrinolysis. However, a number of normotensive patients will develop clinical deterioration, requiring subsequent thrombolysis. Therefore, this has contributed to conclude that size does not matter [29].
A recent meta-analysis assessing the localization of emboli visualized at CT angiography was useful for the stratification of patients [30], though there was no correlation between the obstruction index and prognosis. Another meta-analysis has concluded that the strongest radiological predictive value for adverse outcome in patients with pulmonary embolism is the right to left ventricular ratio measured on CT [31].
However, the analysis of the adverse outcomes using as predictive tools the CT angiography and echocardiography, evaluating the burden and the localization of clots or the overload or dysfunction of the right ventricle, both have been estimated at short-term (i.e. inhospital and 30-days mortality or ICU admission).
To our knowledge there are not studies approaching the long-term prognosis of pulmonary embolism affecting the main pulmonary arteries. Therefore, our aim was to study the prognostic significance of pulmonary embolism affecting pulmonary arteries of different size and to check the survival at long-term differentiating central pulmonary embolism and peripheral pulmonary embolism.
Patients and Methods
In the period 2004-2013, all consecutive outpatients hospitalized on the Internal Medicine Service with a diagnosis of acute symptomatic hemodynamically stable pulmonary embolism, diagnosed by helical chest CT, were evaluated within 24 hours of admission. This study was approved by the local ethics committee. Because the study was observational and did not interfere with diagnostic or therapeutic work-up, informed consents were not obtained. Each patient approved and signed the informed consent for radiologic contrast administration.
Study design and methods
Systematically, we recorded on admission blood pressure, shock index (the ratio of heart rate to systolic blood pressure), heart and respiratory rates, blood gases value before supplementary oxygen administration, electrocardiographic recording, days of symptoms up to diagnosis, and calculated alveolar-arterial difference of oxygen. Alveolar-arterial oxygen gradient was calculated as:
FiO2(Pb-47) _ PACO2/R _ F1O2/R (1-R) (PaCO2/R) _ PaO2
where FIO2 is the O2 inspiratory fraction, Pb is the barometric pressure and PACO2 is alveolar CO2 pressure, PaCO2 is arterial CO2 pressure, assumed to be equal to PCO2, and PaO2 is arterial oxygen pressure. R is the respiratory exchange ratio, set to be 0.8.
Single-slice helical CT was used for diagnosis in 23% patients and multi-detector scanner of 64 rows was used for diagnosis in the rest, both were General Electric devices (Medical Systems, Milwaukee, WI). One mm slices and standard sequential acquisition were obtained in every patient. Breath-hold acquisition was employed. After the intravenous injection of contrast material, the scanning area comprised the chest and upper abdomen, acquiring images in the cranio-caudal direction. Central PE was diagnosed when thrombi were visualized in the main trunk of the pulmonary artery and/or in right or left main pulmonary arteries. Peripheral PE was diagnosed when thrombi were seen exclusively in segmental or sub segmental pulmonary arteries. Each scan was read by a radiologist as in usual clinical practice. Radiologists were blinded to the clinical, laboratory outcomes and survival. Subsequently, the scans were also reviewed by investigators belonging to Internal Medicine Service.
Thrombotic burden was calculated with the formula for the CT obstruction index [32] applied to the initial CT angiography, which was diagnostic of pulmonary embolism. Each lung is considered to have 10 arteries, 3 in the upper lobe, 2 in the middle lobe and lingula and 5 in the lower lobe. The presence of embolus in a segmental artery was scored as 1 point, and emboli in the most proximal arterial level was scored as the value equal to the number of segmental arteries arising distally. A weight factor was assigned depending on the degree of vascular obstruction: 1 point when the thrombus was partially occlusive, and 2 points with total occlusion. Therefore, maximal CT obstruction index is 40 points. The percentage of vascular pulmonary obstruction was calculated as follows: n.d/40×100, where n is the value of the proximal thrombus in the pulmonary arterial tree equal to the number of segmental branches arising distally and d is the degree of obstruction.
The degree of pulmonary obstruction was calculated by the clinicians who were taking care the patients, who were the authors belonging to Internal Medicine. We scored 2 points for the artery where the irrigated territory of a pulmonary infarction was seen and when contrast was not observed distal to the thrombus. The rest of the cases were scored 1 point.
In every patient, blood was drawn within 24 hours of admission for pro-BNP and Troponin I determination. Plasma D-dimer levels were measured previously in the emergency ward.
Standard therapy consisted of enoxaparin 1 mg/kg twice a day for 3 to 5 days, initiation of oral anticoagulants (coumarone) on the first day of hospitalization, overlap of enoxaparin and oral anticoagulants for a minimum of 3 days, and cessation of enoxaparin when INR was greater than 2. During hospitalization fibrinolysis was subsequently indicated in 3 patients due to hemodynamic instability. After treatment with enoxaparin, secondary prophylaxis was made with direct action anticoagulants in 7 patients: Apixaban 2 patients, Rivaroxaban 4 patients and Dabigatran 1 patient.
Death rate was defined as deaths by all causes during hospitalization and those occurred at follow-up. The cause of death by recurrent pulmonary embolism was considered when new thrombotic material in the pulmonary arterial tree was demonstrated either with angio-CT or lung scan and also when the patient had a sudden death with dyspnea.
Cardiovascular death included patients who died because of myocardial infarction, heart failure or reported ventricular dysrrhytmias. Death by all causes was considered in the mortality rate.
Statistical analysis
All continuous variables were tested for normal distribution with the Kolmogorov-Smirnov test. Continuous variables are expressed as median and Interquartile Range (IQR) for variables without normal distribution and as mean ± Standard Deviation (SD) for variable with Gaussian distribution.
Comparison of 2 means was performed with the t test for normally distributed variables and with the Mann-Whitney U test for non-Gaussian variables. Fisher exact test and x2 test were used for proportional comparisons.
Survival analysis was made by using the Mantel-Haenszel test. We tested survival at several times after the index episode in order to see the short, the mid and the long-term survival.
The independence of significant variables obtained from bivariant statistic analysis for central pulmonary embolism was tested with logistic regression by means of a step by step process, eliminating those variables without a level of significance <.05 up to reach of the last useful model. We used standardized coefficient due to the wide variability in measurement units.
All statistical tests were 2-tailed, and a p < 0.05 was considered statistically significant. Values of p greater than 0.05 were considered non-significant.
Results
In the period from January 2004 to December 2013, five hundred and thirty patients consecutively hospitalized because of acute pulmonary embolism were analyzed. Patients were traced during a total time of twelve years.
The median time of follow-up was of 34 (IQR 52) months. The median age was of 76 (IQR 16) years, male 45%. Demographic and baseline data are depicted in Table 1.
Central pulmonary embolism was diagnosed in 255 (48.5%) patients and segmental or sub-segmental (peripheral) thromboembolism in 275 (51.5%) patients. Median age of central pulmonary embolism was 78 (IQR 13) years, while median age of peripheral pulmonary embolism was of 74 (IQR 18) years (p<.001). The concordance between the readings of CT angiography by radiologist and internist doctors was of Kappa 0.87.
Fifty nine (23%) patients with central pulmonary embolism and 56 (20%) patients with peripheral pulmonary embolism had previous cardiac disease (p=.43). Twenty five (10%) patients with central pulmonary embolism and 39 (14%) patients had chronic respiratory disease (p=.11).
Patients with central pulmonary embolism showed a smaller proportion of clinical deep venous thrombosis (28% versus 37% p<.05 CI 95% 0.019-0.17), higher burden of pulmonary thrombi and a higher plasma levels of NT-ProBNP, Troponin I, D-dimer, alveolar to arterial gradient of oxygen, shock index and respiratory rate (p<.001 in each one of the above), while they showed lower arterial partial pressure of oxygen (p<.001), lower arterial partial pressure of carbon dioxide (p<.001) and systolic blood pressure (p<.05) than patients with peripheral pulmonary embolism (Table 1).
All Patients
Central PE
Peripheral PE
p
Number of patients
530
255 (48.5%)
275 51.5%)
Age (years)
76 (IQR 16)
78 (IQR 13)
74 (IQR 18)
<.001
Gender Male
238 (45%)
103 (43%)
134 (52%)
.057
Unprovoked PE
195 (37%)
92 (37%)
103 (37%)
0.92
Previous cancer
65 (12%)
38 (15%)
27 (10%)
0.08
Previous VTE
90 (17%)
43 (16%)
47 (18%)
0.54
DVT clinically evident
175 (33%)
71 (28%)
102 (37%
<.05
Death
176 (33%)
102 (40%)
74 (27%)
<.01
Calculated thrombi burden %
32.5 (IQR 27.5)
48.13 ± 11.77
28.45 ± 12.07
<.001
NT-ProBNP ng/mL
866 (IQR 2971)
2496 (IQR 4581)
311.6 (IQR)1112
<.001
Troponin I ng/mL
0.04 (IQR 0,11)
0.07 (IQR 0.14)
0.02 (IQR) 0.05
<.001
D-dimer ng/mL
3841 (IQR 5354)
4462 (IQR 1124)
3508 (IQR 4450)
<.001
Days up to initial therapy
5 (IQR 8)
5 (IQR 11)
5 (IQR) 8
0.84
Months of anticoagulation
11 (IQR 20)
12 (IQR 25)
10 (IQR) 14
<.05
PaO2 mm Hg
60 (IQR 16)
58 (IQR 17)
63 (IQR) 25
<.001
PaCO2 mm Hg
35 (IQR 8)
33.2 (IQR 6)
36 (IQR)7
<.001
Aa O2 mm Hg
43.75 (IQR 18.2)
47.63 (IQR 18)
39.75 (IQR 17)
<.001
SBP mm Hg
129 (IQR 26)
126 (IQR 30)
130 (IQR 24)
<.05
Heart rate
86 (IQR 25)
89 (IQR 12)
83 (IQR 25)
<.05
Shock index
0.66 (IQR 0.25)
0.7 (IQR 0.30)
0.65 (IQR 0.24)
<.001
Respiratory rate
22 (IQR12)
24 (IQR 10)
20 (IQR 8)
<.001
% INR of prothrombin >2
75 (IQR 29)
75 (IQR 24)
75 (IQR 30)
0.44
Permanent anticoagulant therapy
217 (41%)
110 (43%)
107 (39%)
0.32
Bleeding
21 (4%)
10 (4%)
11 (4%)
0.96
Cava filter
12 (2%)
6 (2%)
6 (2%)
0.91
VTE: Venous thromboembolism
PE: Pulmonary embolism
DVT: Deep venous thrombosis
AaO2: Alveolar to arterial difference of oxygen
Table 1: Demographic, baseline characteristics and differential characteristics between central and peripheral pulmonary embolism.
Bleeding occurred in 21 (4%) patients. Brain hemorrhage in 10 patients (central pulmonary embolism in 6 and peripheral pulmonary embolism in 4 patients, p=.53), gastrointestinal hemorrhage in 6 patients (central pulmonary embolism in 1 patient and peripheral pulmonary embolism in 5 patients, p=.21), and one retroperitoneal hemorrhage, one muscle hemorrhage and one hematuria requiring blood transfusion in central pulmonary embolism, and 2 hematuria in peripheral pulmonary embolism.
During follow-up 102 (40%) patients with central pulmonary embolism at the index episode died, while 74 (27%) patients who had a segmental or sub segmental pulmonary embolism died (p <.01 CI 95% 0.04-0.21), odds ratio 1.81 (CI 95% 1.16-1.9).
The median time up to death of patients who had central pulmonary embolism was of 19.5 (IQR 52) months after the episode of pulmonary embolism. The median time up to death in patients with segmental or sub segmental pulmonary embolism was of 11.62 (IQR 31.9) months (p=.14). We show in Table 2 mortality at different time from the initial episode.
Time
Overall
Central PE
Peripheral PE
p
15-day
19 (3.5%)
14 (5%)
5 (2%)
=.025
30-day
28 (5%)
16 (6%)
12 (4%)
=0.3
3-month
50 (9.5%)
31 (12%)
19 (7%)
=.03
2-year
105 (20%)
61 (24%)
44 (16%)
=.02
3-year
120 (23%)
67 (26%)
53 (19%)
=.05
5-year
143 (27%)
82 (32%)
61 (22%)
=.009
Table 2: Mortality at different time in patients with central or peripheral pulmonary embolism.
The analysis of survival curves showed a longer survival in patients with segmental and sub-segmental pulmonary thrombi than in patients with central pulmonary both at 10 months (p=.03), at 26 months (p=.03) and at 96 months (p=.0005). When we adjusted the survival curves for patients without previous cardiac and respiratory disease and cancer we observed that the survival continued being better in segmental or sub-segmental pulmonary embolism than in central pulmonary embolism (p=.018) (Figure 1).
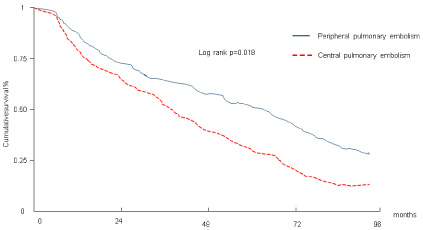
Figure 1: Survival in patients with central and segmental and subsegmental
pulmonary embolism.
The curves of survival shown have been adjusted for confounders: patients
with cardiac, respiratory disease or cancer have not been included in the
analysis.
The thrombi burden of dead patients was of 33.75% (IQR 25) while the thrombi burden of survivors was of 30% (IQR 32.25) (p<.001).
Fifty four patients died while they were with anticoagulant therapy, 34 (33%) belonging to the group of central pulmonary embolism and 21 (28%) belonging to the group of peripheral pulmonary embolism (p=.48). The anticoagulant therapy had been discontinued in 40 (39%) dead patients with central pulmonary embolism 7 ± 5 months after the initial episode and in 31 (42%) dead patients with peripheral pulmonary embolism 6.7 ± 4.52 months after the initial episode (p= .72 and p=.21 respectively). In Table 3 are shown the causes of death, globally and separated by groups of central or peripheral pulmonary embolism.
All patients
Central PE
Peripheral PE
p
Pulmonary embolism
27 (15%)
18 (7%)
9 (3%)
<.05
Cancer
39 (22%)
29 (11%)
10 (4%)
<.001
Cardiovascular death
32 (18%)
18 (7%)
14 (5%)
.34
Bleeding
9 (5%)
8 (3%)
1 0.4%)
<.05*
Stroke
3 (2%)
2 (1%)
1 (0.4%)
.61*
Pneumonia
28 (16%)
17 (7%)
11 (4%)
.17
Sepsis
11 (6%)
2 (1%)
9 (9%)
.06*
COPD
7 (5%)
1 (0.4%)
6 (2%)
.12*
IPD
3 (2%)
0 (0%)
3 (1%)
.24*
Other causes
6 (3%)
1 (0.4%)
5 (2%)
.21*
Unknown
11 (6%)
6 (2%)
5 (2%)
.76*
PE: Pulmonary embolism
Peripheral pulmonary embolism includes segmental and sub-segmental PE
*Two tail Fisher test
COPD: Chronic obstructive pulmonary disease
IPD: Interstitial pulmonary disease
Table 3: Causes of death classified by central or segmental and sub-segmental (peripheral) pulmonary embolism.
At the follow up, patients dead because of a recurrent pulmonary embolism were 18 (7%) patients belonging to the group of central pulmonary embolism and 9 (3%) patients belonging to the group of peripheral pulmonary embolism (p<.05 CI 95% 0.003-0.07).
When the initial episode was a central pulmonary embolism, the patients died because of a recurrent pulmonary embolism at a median time of 0.28 (IQR 13) months, while patients who had had peripheral pulmonary embolism died because of a recurrent pulmonary embolism 18 (IQR 46) months later (p=.12).
Deaths caused by recurrent pulmonary embolism occurred in 12 (40%) patients with permanent anticoagulation which had a median value of prothrombin in therapeutic range of 61.5%, and in 15 (60%) patients who had been withdrew anticoagulation.
Twenty nine patients (11%) with central pulmonary embolism and 10 (4%) patients with peripheral pulmonary embolism both at the index episode died because of different cancers (p<.001 CI 95% 0.02-0.12). Deaths by cancer in patients with central pulmonary embolism occurred 19 (IQR 40) months after the initial episode and 6.6 (IQR 33) months in those patients with peripheral pulmonary embolism (p non significant).
In Table 4 we show the results of logistic regression analysis. Independent variables predicting the death were the age of the patient at the index episode (OR 2.89 CI 95% 1.04-1.10), the development of cancer during the follow-up of the patient (OR 1.48 CI 95% 1.64- 7.71), the central thrombi at the index episode (OR 1.31 CI 95% 1.007-3) and the plasma level of NT-ProBNP measured at the index episode (OR 1.61 CI 95% 1.0001-1.0002). Respiratory rate at the index episode was not an independent predictive variable of death.
ß
p
Odds ratio
CI 95%
Age
1.06
.00001
2.89
1.04-1.10
Cancer diagnosed during follow-up
0.39
.001
1.48
1.64-7.71
Central thrombi
0.27
.04
1.31
1.007-3
NT-ProBNP
0.48
.002
1.61
1.001-1.002
Respiratory rate
0.22
.09
1.23
0.99-1.07
Table 4: Logistic regression of variables predicting death.
Discussion
Despite the localization of pulmonary emboli into the pulmonary arterial tree is not currently considered a fact of severity of the pulmonary embolism, there are several studies supporting the fact that the closer they are to the right ventricle the earlier and higher is the short-term mortality [22-25], while emboli affecting small pulmonary arteries carry a better prognosis [24]. However, not all studies have shown a direct relationship between the size of the occluded vessel and mortality, with several investigations that included a moderate number of patients [26-28] unable to show correlation between image and prognosis.
In the same way, the arterial obstruction index has shown to be useful in several investigations in order to predict right ventricular dysfunction and death, although in a recent meta-analysis, despite the fact of localization of pulmonary emboli assessed by computed tomography angiography showed usefulness for risk stratification, the obstruction index did not show relation with the prognosis [30].
In our patients, the central localization of emboli respect to segmental or sub segmental emboli was associated with more stress of right ventricle measured with higher plasma levels of NT-ProBNP and troponin I and a more intense disorder in gas exchange and hemodynamic status.
The clot burden was also higher in central pulmonary embolism than in segmental and sub segmental pulmonary embolism. However, this fact seems derived from the characteristic of the equation for calculating the clot burden, since it could not demonstrate whether it is an independent factor in the prediction of death.
Patients with segmental and sub segmental pulmonary embolism had more clinically overt signs of deep venous thrombosis than patients with central pulmonary embolism. This fact could be explained by migration of thrombi from lower limbs to the pulmonary circulation in patients with central pulmonary embolism showing a higher thrombi burden. A defective fibrinolytic system joined to a higher degree of hypoxemia and activation of inflammatory pathways could also interact favoring the greater size of emboli, although in our patients a higher plasma level of D-dimer goes against quantitative defects in fibrinolysis.
On the other hand, our patients are mostly elderly and the age of patients with central pulmonary embolism was higher than the age of patients with segmental and sub segmental pulmonary embolism. In this way, a defective fibrinolysis and endothelial function has been showed in the elderly, and so all this factors could contribute to the higher size of emboli [33], which would cause the lodging of thrombi in the proximal pulmonary arteries.
In our study patients with central lodged thrombi showed a higher overall mortality than patients with more peripheral pulmonary embolism, with more mortality rate specifically due to subsequent pulmonary embolism, cancer and bleeding. However, neither the time of anticoagulant therapy of patients with central and more peripheral pulmonary embolism nor the proportion of patients dead while they were under anticoagulant therapy were different enough as to explain the higher mortality of central pulmonary embolism. The number of patients with direct action anticoagulants is too small to analyze and to draw valid conclusions.
Survival showed in patients with central pulmonary embolism was significantly lower than in patients with segmental or sub segmental thrombi. Sub-analysis at different times from the initial episode also demonstrated an increased mortality for central pulmonary embolism at short (i.e. 10 months), mid (i.e. 26 months) and longterm (i.e. 96 months).
In our patients, the in-hospital mortality rate measured at 15-day and 30-day is lower than the mortality reported in literature, which has been estimated ranging between 9-11% at 30-day and between 8,6-17% at 3-months [34-37]. The high long-term mortality in our study (33%) may be explained in part by the advanced age of our patients.
Variables such as gas exchange data, hemodynamic values, the plasma level of troponin I, the clot burden and the absence of overt signs of deep venous thrombosis disappeared from the model of logistic regression on losing significance.
In the final model, independent variables predicting death were the age of the patient, the plasma level of NT-ProBNP, both measured at index episode, and the development of cancer during the follow up of the patients, while the segmental or sub segmental pulmonary embolism was a protective factor. In the final model the respiratory rate remained although it did not show significance.
However, our study has several limitations. The patients were drawn from a single centre; therefore our results should be tested in other studies or in meta-analysis. Although radiologists that interpreted the computed tomography angiography were blinded for the study, different assessments of the localization of thrombi made by them could be due to the fact that radiologists on duty not always are specialized in thorax radiology. Thereafter, the review of the scans for the authors belonging to internal medicine produced a high level of concordance playing down the potential bias. Another limitation of our study is that the death of a number of patients occurred because of unknown causes, although the number was similar in both groups minimizing the impact over the other causes of death.
Another potential limitation of our study could be due to an overestimation of deaths caused by recurrent pulmonary embolism, since sudden deaths were included as recurrent pulmonary embolism and they might have occurred by other causes, such as ventricular arrhythmia.
Patients of this study are mostly hemodynamically stable with a few patients needing subsequent fibrinolysis. Thereafter, our results cannot be extrapolated to patients with hemodynamic instability, only to patients who meet criteria for sub-massive or non-massive pulmonary embolism.
A potential strength of our study is the fact of to have been carried out in a relatively closed community. This fact has allowed a close follow-up of the patients while no patients have been lost.
Conclusion
The patients with hemodynamically stable acute pulmonary embolism which show thrombi lodged in the main pulmonary arteries have a higher overall mortality and lower survival than patients with segmental or sub segmental pulmonary embolism.
References
- Araoz PA, Gotway MB, Trowbridge RL, Bailey RA, Auerbach AD, Reddy GP, et al. Helical CT pulmonary angiography predictors of in-hospital morbidity and mortality in patients with acute pulmonary embolism. J Thorac Imaging. 2003; 18: 207-216.
- van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005; 235: 798-803.
- Moroni AL, Bosson JL, Hohn N, Carpentier F, Pernod G, Ferretti GR. Non-severe pulmonary embolism: prognostic CT findings. Eur J Radiol. 2011; 79: 452-458.
- Bazeed MF, Saad A, Sultan A, Ghanem MA, Khalil DM. Prediction of pulmonary embolism outcome and severity by computed tomography. Acta Radiol. 2010; 51: 271-276.
- Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN. Pulmonary embolism: prognostic CT findings. Radiology. 2007; 242: 889-897.
- Díaz JC, Ladrón de Guevara D, Pereira G, Herrmann R, Silva C, Astorga E, et al. Medición de la carga embólica y relación ventrículo derecho/ventrículo izquierdo en tomografía computada como indicador de riesgo en pacientes con tromboembolismo pulmonar. Rev Med Chile. 2007; 135: 1437-1445.
- Nural MS, Elmali M, Findik S, Yapici O, Unzun O, Sunter AT, et al. Computed tomographic pulmonary angiography in the assessment of severity acute pulmonary embolism and right ventricular dysfunction. Acta Radiol. 2009; 50: 629-637.
- Engelke C, Rummeny E, Marten K. [Acute pulmonary embolism: prediction of cor pulmonale and short-term patient survival from assessment of cardiac dimensions in routine multidetector-row CT]. Rofo. 2006; 178: 999-1006.
- Ghaye B, Ghuysen A, Willems V, Lambermont B, Gerard P, D'Orio V, et al. Severe pulmonary embolism:pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology. 2006; 239: 884-891.
- Becattini C, Agnelli G, Vedovati MC, Pruszczyk P, Casazza F, Grifoni S, et al. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J. 2011; 32: 1657-1663.
- Kang DK, Thilo C, Schoepf UJ, Barraza JM Jr, Nance JW, Bastarrika G, et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011; 4: 841-849.
- Baptista R, Santiago I, Jorge E, Teixeira R, Mendes P, Curvo-Semedo L, et al. One-shot diagnostic and prognostic assessment in intermediate-to high-risk acute pulmonary embolism: the role of detector computed tomography. Rev Port Cardiol. 2013; 32: 7-13.
- Trujillo-Santos J, den Exter PL, Gómez V, Del Castillo H, Moreno C, van der Hulle T, et al. Computed tomography- assessed right ventricular dysfunction and risk stratification of patients with acute non-massive pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013; 11: 1823-1832.
- Wu AS, Pezzullo JA, Cronan JJ, Hou DD, Mayo-Smith WW. CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome--initial experience. Radiology. 2004; 230: 831-835.
- Rodrigues B, Correia H, Figueiredo A, Delgado A, Moreira D, Ferreira Dos Santos L, et al. [Clot burden score in the evaluation of right ventricular dysfunction in acute pulmonary embolism: quantifying the cause and clarifying the consequences]. Rev Port Cardiol. 2012; 31: 687-695.
- Zhou Y, Shi H, Wang Y, Kumar AR, Chi B, Han P. Assessment of correlation between CT angiographic clot load score, pulmonary perfusion defect score and global right ventricular function with dual-source CT for acute pulmonary embolism. Br J Radiol. 2012; 85: 972-979.
- Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012; 265: 283-293.
- Aviram G, Rogowski O, Gotler Y, Bendler A, Steinvil A, Goldin Y, et al. Real-time risk stratification of patients with acute pulmonary embolism by grading the reflux of contrast into the inferior vena cava on computerized tomographic pulmonary angiography. J Thromb Haemost. 2008; 6: 1488-1493.
- Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care. 2011; 15: R103.
- Alonso-Martínez JL, Urbieta-Echezarreta M, Anniccherico-Sánchez FJ, Abínzano-Guillen ML, García-Sanchotena JL. N-Terminal Pro-B-Type Natriuretic peptide predicts the burden of pulmonary embolism. Am J Med Sci. 2009; 337: 88-92.
- Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35: 3033-3069, 3069a-3069k.
- Klok FA, Djurabi RK, Nijkeuter M, Eikenboom HC, Leebeek FW, Kramer MH, et al. High D-dimer level is associated with increased 15-d and 3 months mortality through a more central localization of pulmonary emboli and serious comorbidity. Br J Haematol. 2008; 140: 218-222.
- Venkatesh SK, Wang SC. Central clot score at computed tomography as a predictor of 30-day mortality after acute pulmonary embolism. Ann Acad Med Singapore. 2010; 39: 442-447.
- Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010; 126: 266-270.
- Vedovati MC, Becattini C, Agnelli G, Kamphuisen PW, Masotti L, Pruszczyk P, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012; 142: 1417-1424.
- Nakada K, Okada T, Osada H, Honda N. Relation between pulmonary embolus volume quantified by multidetector computed tomography and clinical status and outcome for patients with acute pulmonary embolism. Jpn Radiol. 2010; 28: 34-42.
- Ceylan N, Tasbakan S, Bayraktaroglu S, Cok G, Simsek T, Duman S, et al. Predictors of clinical outcome in acute pulmonary embolism: Correlation of CT pulmonary angiography with clinical, echocardiography and laboratory findings. Acad Radiol. 2011; 18: 47-53.
- Soares TH, de Bastos M, de Carvalho BV, Moreira W, Cabral CP, de Paula LF, et al. Prognostic value of computed tomographic pulmonary angiography and the pulmonary embolism severiti índex in aptients with acute pulmonary embolism. Blood Coagul Fibinolysis. 2013; 24: 64-70.
- Elliott CG. Fibrinolysis of pulmonary emboli--steer closer to Scylla. N Engl J Med. 2014; 370: 1457-1458.
- Vedovati MC, Germini F, Agnelli G, Becattini C. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013; 11: 2092-2102.
- Meinel FG, Nance JW Jr, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, et al. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am J Med. 2015; 128: 747-759.
- Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001; 176: 1415-1420.
- Leurs PB, Stolk RP, Hamulyak K, Van Oerle R, Grobbee DE, Wolffenbuttel BH. Tissue factor pathway inhibitor and other endothelium-dependent hemostatic factors in elderly individuals with normal or impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2002; 25: 1340-1345.
- Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006; 166: 169-175.
- Laporte S, Mismetti P, Décousus H, Uresandi F, Otero R, Lobo JL, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad Tromboembólica Venosa. Circulation. 2008: 117: 1711-1716.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999; 353: 1386-1389.
- Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: Case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010; 152: 578-589.