
Case Report
Austin J Pulm Respir Med 2015; 2(3): 1030.
Lung Cancer of Unknown Primary Site with EGFR Mutation as Indicated by Frozen Bone Specimen
Boku S¹*, Takase N¹, Onoe T¹, Shibata Y¹, Matsumoto K¹, Satouchi M², Sakuma T³, Sudo T4 and Negoro S¹
¹Department of Medical Oncology, Hyogo Cancer Center, Japan
²Department of Thoracic Oncology, Hyogo Cancer Center, Japan
³Department of Diagnostic Pathology, Hyogo Cancer Center, Japan
4Section of Translational Research, Hyogo Cancer Center, Japan
*Corresponding author: Shogen Boku, Department of Medical Oncology, Hyogo Cancer Center, 13-70 Kitaojicho, Akashi-City, Hyogo 673-8558, Japan
Received: September 25, 2015; Accepted: November 02, 2015; Published: November 03, 2015
Abstract
We reported a case of lung cancer of unknown primary site harboring EGFR mutation revealed in frozen bone specimens. Tumor cells obtained from bone metastasis are associated with a higher rate of molecular testing failure. We submitted the preserved frozen bone specimens and detected EGFR exon 19 deletions. She has achieved long-term disease-free survival from gefitinib. This result shows the importance of gene analysis of frozen bone specimens in CUP with bone metastases.
Keywords: Lung cancer; EGFR mutation; Bone metastasis; Gefitinib
Case Report
A 75 year-old Japanese women was referred to our hospital complaining of left-sided chest pain. Her performance status (ECOG) was 1. She was a never smoker. On physical examination, there was no palpable mass on her trunk or extremities. Blood examination showed significant elevation of Carcinoembryonic Antigen (CEA) to 209.5 ng/ml. Chest-X rays and Computed Tomography (CT) did not detect the primary lesion in her lung or pleura. Positron Emission Tomography and Computed Tomography (PET-CT) detected abnormal FDG uptake of left 5th and 6th rib and subaortic (Botallo’s) lymph nodes (standardized uptake value-max 5.0, Figure 1A). We performed open biopsy of her left 5th rib in order to ascertain malignancy. We performed decalcification, and some of the samples were H&E (hematoxyline-eosin)-stained. We fixed the rest of the specimens in liquid nitrogen and kept them at -70oC in a deep freezer to be used in any future genetic testing. The HE staining showed the tumor cells to be adenocarcinoma. Immunohistochemical testing showed that the tumor cells to be positive for Thyroid Transcription Factor-1 (TTF-1), Napsin A, and CAM5 2 (Figure 2). Our opinion was that this bony malignancy derived from lung. To examine the gene mutations, we submitted the preserved frozen specimens to the laboratory cooperation (LSI Medience Corporation) and requested processing with protease. EGFR exon 19 deletions were detected by PNA-LNA PCR clamp. We started the patient on gefitinib and bonemodifying agent (zoledolnic acid) in Feb 2013. Skin rash associated with gefitinib was observed on her legs. It was tolerable without dose reduction or discontinuation. Serum levels of CEA normalized four months after she received gefitinib. The PET-CT at the 24th month did not detect the signs of recurrence or progression in terms of either bone metastasis or mediastinal lymph nodes (Figure 1B).
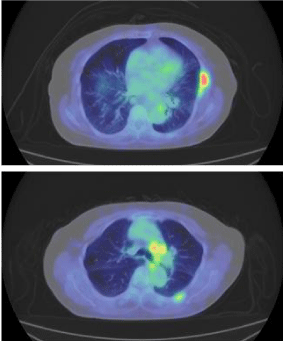
Figure 1a: PET-CT detected abnormal FDG uptake of left rib, and aortic-arch
lymph nodes.
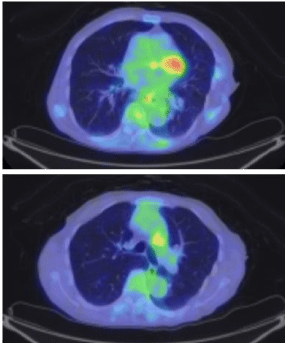
Figure 1b: Lower FDG uptake was detected in both lesions 24 months after
she received gefitinib. (max SUV 5.0→1.6).
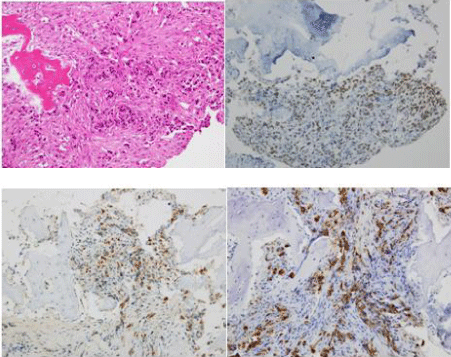
Figure 2: Tumor specimens obtained from bony tissue (a. HE, b. TTF-1, c.
Napsin, d. CAM5.2).This result of immunostaining presented a phenotype of
lung cancer. (All images were taken at 200× original magnification).
Discussion/Conclusion
We reported a case of lung cancer of unknown primary site harboring EGFR mutation revealed in frozen bone specimens. The pathological examination suggested lung cancer. The present case characteristics include Asian, female, a history of non-smoking, and histologic evidence of adenocarcinoma. These characteristics are highly associated with Non-Small Cell Lung Cancer (NSCLC) harboring EGFR mutation [1]. Tumor cells obtained from bone metastasis, however, are associated with a higher rate of molecular testing failure [2]. The routine processing of bone specimens incorporates an acid decalcification step. Acid decalcification of bone tissue tends to cause extensive fragmentation of DNA and also interferes with subsequent genetic analysis [3]. We showed that preserved frozen bone specimens were useful for genetic testing and decision making in treatment. We routinely preserved frozen specimens obtained from patients for whom there was a suspicion of Cancer of Unknown Primary (CUP) in our institution. This technique is not common in general hospitals because it is difficult to obtain liquid nitrogen. Recently it was reported that using process of EDTA decalcification or no decalcification, DNA quality was high and sequences could be easily analyzed on bone metastases specimens [4]. PNA-LNA PCR clamp can detect EGFR mutations in the presence of 100-to 1,000-fold background of wild-type EGFR [5]. This system can accurately detect EGFR mutations as much as direct sequencing, Cycleave™, and Scorpion Amplification Refractory Mutation System (ARMS)®in the analysis of FFPE and cytology samples [6]. In NSCLC harboring EGFR mutations, gefitinib is feasible for elderly patients or patients with poorer PS [7]. Therefore, we selected gefitinib as first-line therapy for this patient. She has achieved long-term diseasefree survival from gefitinib. It was reported that bone metastases and skeletal related events were more frequent in men, heavy smokers and without treatment of EGFR TKI. The presence of bone metastases proved to be an independent prognostic factor related to poor performance status and worse Quality of Life [8]. This result shows the importance of gene analysis of frozen bone specimens in CUP with bone metastases.
References
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). Journal of clinical oncology. 2011; 29: 2866-2874.
- Vanderlaan PA, Yamaguchi N, Folch E, Boucher DH, Kent MS, Gangadharan SP, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014; 84: 39-44.
- Baloglu G, Haholu A, Kucukodaci Z, Yilmaz I, Yildirim S, Baloglu H. The effects of tissue fixation alternatives on DNA content: a study on normal colon tissue. Applied immunohistochemistry & molecular morphology. 2008; 16: 485-492.
- Confavreux CB, Girard N, Pialat JB, Bringuier PP, Devouassoux-Shisheboran M, Rousseau JC, et al. Mutational profiling of bone metastases from lung adenocarcinoma: results of a prospective study (POUMOS-TEC). Bonekey Rep. 2014; 3: 580.
- Nagai Y, Miyazawa H, Huqun, Tanaka T, Udagawa K, Kato M, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005; 65: 7276-7282.
- Goto K, Satouchi M, Ishii G, Nishio K, Hagiwara K, Mitsudomi T, et al. An evaluation study of EGFR mutation tests utilized for non-small-cell lung cancer in the diagnostic setting. Ann Oncol. 2012; 23: 2914-2919.
- Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. Journal of clinical oncology. 2009; 27: 1394-1400.
- Bittner N, Balikó Z, Sárosi V, László T, Tóth E, Kásler M, et al. Bone Metastases and the EGFR and KRAS Mutation Status in Lung Adenocarcinoma - The Results of Three Year Retrospective Analysis. Pathol Oncol Res. 2015; 21: 1217-1221.