
Editorial
Austin J Pulm Respir Med 2015; 2(3): 1032.
Extracorporeal Membrane Oxygenation Bridge to Lung Transplantation in Patients with Cystic Fibrosis
Kamyar Afshar¹* and Eugene Golts²
¹Division of Pulmonary, Critical Care and Sleep Medicine, University of California, USA
²Division of Cardiothoracic Surgery, University of California, USA
*Corresponding author: Kamyar Afshar, Division of Pulmonary, Critical Care and Sleep Medicine, University of California, San Diego School of Medicine, 9300 Campus Point Drive, Mail Code 7376 La Jolla, CA 92037, USA
Received: November 10, 2015; Accepted: November 13, 2015; Published: November 16, 2015
Editorial
The progressive deterioration of respiratory status in patients with cystic fibrosis may produce hypoxemia, hypercapnea and eventual respiratory failure. Lung transplantation is a therapeutic option for selected patients with end stage lung disease from cystic fibrosis that continue to have progressive clinical deterioration despite maximal medical therapies. The appropriate timing to refer a patient with cystic fibrosis for transplantation is based on several considerations. This includes patient prognosis, projected estimates of survival time following transplantation and the risk of mortality while on the waiting list. The goal is to get a lung transplant while within the “transplant window”. This “window” is the period when the patient is ill enough that transplantation becomes a clinical option, but not too ill when the risk of the surgery outweighs any clinical benefit.
Once deemed a suitable candidate, the time on the waiting list varies based on geographical transplant program regions, ABO blood type, presence of donor specific antibodies; HLA matching and donor/ recipient size matches. One of the most important factors though is the dependent on donor lung availability. The overall median waitlist time for lung transplantation is less than 6 months.
Despite appropriate and timely referral for lung transplantation, the natural progression of lung disease in individuals with cystic fibrosis does not halt. Eventually, the recurrent inflammatory or infectious process increased dead-space ventilation with filling of exudative fluid management and ventilation-perfusion mismatches results in the need for invasive mechanical ventilation in a selective group of patients with cystic fibrosis.
The invasive mechanical ventilation itself limits effective airway clearance and results in the perpetual retention of the thick airway secretions. Even therapeutic suctioning via a flexible bronchoscopy can worsen the V/Q mismatch with removal of surfactants with the suctioning.
Candidates are selected based on the likelihood of a successful outcome. This group faces two overwhelming competing challenges when transitioning to lung transplantation.
First, when requiring mechanical ventilator support for respiratory failure for an acute infectious exacerbation, cystic fibrosis patients have a significantly high mortality (up to 74%) [1,2]. The goal is to obtain suitable donors quickly. The Lung Allocation Score (LAS) implementation has helped determine that more critically ill candidates receive lung transplantation earlier. The LAS is not the only determinant to receive lung transplantation within the “transplant window”. For example, candidates with LAS greater than 50 have a median wait time of 1 month. Depending on the region, the time to lung transplantation may take up to 2-3 months even for ventilated dependent patients and have high lung allocation score. This may be a tolerable timeline for a minority of patients. On an annual basis; however, up to 28% of lung transplant candidates will be either inactivated or die before an adequate lung donor becomes available [3].
Concurrently, ventilator dependence is a recognized risk factor for poor survival after transplantation [4]. Many transplant centers are willing to keep ventilated dependent patients on the active wait list for a brief period (less than 2 weeks) without signs of extra-pulmonary organ damage. Longer periods will warrant demonstrating active physical rehabilitation. Even in situations where a candidate with high LAS undergoes transplantation in a relative short time after listing, this may be offset by overall survival [5,6]. Lung transplant candidates having LAS > 60 (20-44 days) have been compared to candidates with LAS < 60 (55-98 days) [6]. Lung transplant candidates with LAS > 60 had all of the following post lung transplantation: 1) a greater need for post-operative mechanical ventilation, 2) higher rate of ICU admission, 3) longer immediate hospital course, 4) higher incidence of graft failure (13% vs. 3%) and 5) worse 1-year survival (83.7% for LAS < 46 and 68-75% for LAS > 60).
In specialized centers, Extracorporeal Membrane Oxygenation (ECMO) has been a utilized procedure to allow patients to remain suitable lung transplant candidates.
ECMO provides support for both hypoxemia and hypercapnea in refractory states while receiving invasive mechanical ventilation. Two types of ECMO support are widely used – Veno-Arterial (VA) and Veno-Venous (VV). Both types involve intake of deoxygenated blood from the systemic venous circulation. Blood is subsequently passed through the membrane oxygenator with the help of the pump and returned to the patient. Blood return is to arterial side of systemic circulation in veno-arterial configuration and to the venous side of systemic circulation in the veno-venous configuration. VV ECMO provides oxygenation and removal of carbon dioxide. VA ECMO provides the same gas exchange opportunities, but additionally provides circulatory system support. Of these two, VV ECMO is uniquely suited to support cystic fibrosis patients in a refractory state on the mechanical ventilator for respiratory failure.
Traditionally, the need for two cannulas in either configuration prevented patients from being physically active. Newer, single catheter, dual-lumen venous cannula utilized for VV ECMO support allows sedatives to be minimized and weaned off as well as extubation from the mechanical ventilation, thereby promoting rehabilitation with physical therapy. Major advances to the technical proficiencies, circuit types and development of membrane-type oxygenators are important considerations between the various eras of practice have most likely helped with the overall improvement in survival with ECMO [7-9].
The benefits of using ECMO specifically in patients with CF have been analyzed by Hayes and colleagues [10]. They retrospectively reviewed the data on the use of ECMO for patients with cystic fibrosis between January 1998 and April 2003. The overall survival for the cohort (including 8 CF patients on a combined VV and VA ECMO) was 52%. When they compared the survival of 33 CF patients receiving VV ECMO to 32 patients receiving VA ECMO, there was a slight preference towards the VV ECMO (56% VV ECMO vs. 44% VA ECMO; p = NS). Important limitations for this analysis include limited data reporting from the Extracorporeal Life Support Organization (ESLO) Registry and the lack of comparison between different eras. It is well established that there are conflicting outcomes in the research and clinical care in this specific field.
A more recent ESLO registry query of 91 CF transplant related cases on ECMO from 1998 to mid-2012 show that survival has improved after 2007 [11]. Before 2007, the survival rate was 45%. After 2007, it improved to 70%. Poor prognostic factors included sepsis and time to initiate ECMO less than 2 days after initiating mechanical ventilation. The length of ECMO run for the survivors was 10.3 days (IQR 5.6 – 17.9 days) compared to 8 days for the nonsurvivors (IQR 2.9 – 18.1 days).
Even more recent description of cases describe improved outcome in CF patients requiring VV ECMO as a bridge to lung transplantation [11-13]. Abu-Sultaneh et al. describe the use of a “Daily Lung Compliance Trial” (DLCT) for 19 year-old woman with cystic fibrosis who was intubated for respiratory failure and hemodynamic compromise from septic shock requiring VV ECMO [11]. “DLCT” was initiated on day 6 of VV ECMO. The “DLCT” consisted of a temporary increase in the mechanical ventilator settings from a “resting mode” (SIMV-PC, FiO2 = 0.5, Rate = 8, iT = 1.2 seconds, PEEP = 12, PIP = 26) to a “moderate setting” (SIMVPRVC FiO2 = 0.5, Vt = 6 cc/kg, Rate = 8, iT = 1.2 seconds, PEEP = 12) for 30 minutes. At the end of the “DLCT”, PIP, plateau pressure, mean airway pressure, arterial blood gas, oxygenation index, static compliance and dynamic compliance were calculated Figure 1 illustrates improvement in objective parameters to determine when VV ECMO may be successfully discontinued.
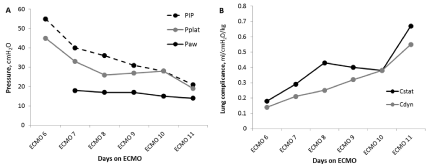
Figure 1: Daily lung compliance trial from Day 6 through Day 11 VV ECMO. (A) Peak, plateau and mean airway pressures and (B) lung compliance. Cdyn: Dynamic
Compliance; Cstat: Static Compliance; Paw: Mean Airway Pressure; PEEP: Positive End-Expiratory Pressure; PIP: Peak Inspiratory Pressure; Pplat: Plateau
Pressure [11].
Reeb et al. have reported the initiation of VV ECMO via double lumen bi-cava cannula in a non-intubated cystic fibrosis awaiting lung transplantation [13]. The 35 year-old man with cystic fibrosis and refractory hypercapnea (pH = 7.10, PaCO2 = 103 mmHg, PaO2 = 83 mmHg) on a non-invasive mechanical ventilator was successfully transitioned to lung transplant after 11 days of VV ECMO with via 27 French double lumen bi-cava cannula (Avalon Elite™). It was reported that the patient was able to speak, eat and drink, practice both active and passive physiotherapy and receive psychological support.
ECMO is associated with significant complications. Bleeding, access site/extremity complications, gas and particle embolism, infection, and thrombosis are the most feared complications of this treatment modality.
Coagulation and fibrinolytic cascades are activated simultaneously upon contact of the patient’s blood with the circuit surfaces and oxygenator and action of the pump head. Aggressive anticoagulation protocols predispose patients to bleeding in both the preoperative and intraoperative period. Perioperative bleeding is of particular concern as cystic fibrosis patients frequently have significant amount of pleural adhesions. Takedown of these pleural adhesions and enlarged tortuous bronchial arterial collateral vessels coupled with pre-operative anticoagulation and ECMO-induced fibrinolysis can turn recipient pneumonectomies into rather bloody operations. Some experts suggest that operations on the patients maintained on ECMO are associated with prohibitive risk of bleeding [14]. Our recent experience is similar to many other lung transplant centers in that lung transplant surgeries can be safely performed on the patients supported by ECMO pre-operatively.
Traditionally, systemic anticoagulation is administered to all patients on an ECMO system. Recently reports have emerged that VV ECMO can be safely performed without administration of anticoagulation therapy in patients with high risk for bleeding [15,16]. This novel approach certainly merits attention when initiating ECMO in the pre-operative setting.
The use of extracorporeal membrane oxygenation as a bridge to lung transplantation for patients with cystic fibrosis appears to be promising. This technology will most likely gain traction in assisting patients prolong life and allow them to remain suitable lung transplant candidates while minimizing the risks with the ECMO technology.
References
- Ellaffi M, Vinsonneau C, Coste J, Hubert D, Burgel PR, Dhainaut JF, et al. One-year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005; 171: 158-164.
- Efrati O, Bylin I, Segal E, Vilozni D, Modan-Moses D, Vardi A, et al. Outcome of patients with cystic fibrosis admitted to the intensive care unit: is invasive mechanical ventilation a risk factor for death in patients waiting lung transplantation? Heart Lung. 2010; 39: 153-159.
- Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006; 6: 1212-1227.
- Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg. 2010; 139: 765-773.
- Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, et al. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant. 2009; 28: 769-775.
- Liu V, Zamora MR, Dhillon GS, Weill D. Increasing lung allocation scores predict worsened survival among lung transplant recipients. Am J Transplant. 2010; 10: 915-920.
- Jurmann MJ, Schaefers HJ, Demertzis S, Haverich A, Wahlers T, Borst HG. Emergency lung transplantation after extracorporeal membrane oxygenation. ASAIO J. 1993; 39: M448-452.
- Fischer S, Simon AR, Welte T, Hoeper MM, Meyer A, Tessmann R, et al. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg. 2006; 131: 719-723.
- Fischer S, Hoeper MM, Tomaszek S, Simon A, Gottlieb J, Welte T, et al. Bridge to lung transplantation with the extracorporeal membrane ventilator Novalung in the veno-venous mode: the initial Hannover experience. ASAIO J. 2007; 53: 168-170.
- Hayes D Jr, Kopp BT, Preston TJ, Kirkby S, Tobias JD, Papadimos TJ, et al. Survival of patients with cystic fibrosis on ECMO: analysis of the Extracorporeal Life Support Organization Registry. Int J Clin Exp Med. 2014; 7: 1370-1372.
- Abu-Sultaneh S, Raj SS, Benneyworth BD, Cristea AI, Nitu ME, Rigby MR. Use of a daily lung compliance evaluation to track progress on ECMO: Successful use in a patient with cystic fibrosis. J Med Cases. 2014; 5: 83-88.
- Hayes D Jr, Kukreja J, Tobias JD, Ballard HO, Hoopes CW. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros. 2012; 11: 40-45.
- Reeb J, Falcoz PE, Santelmo N, Massard G. Double lumen bi-cava cannula for veno-venous extracorporeal membrane oxygenation as bridge to lung transplantation in non-intubated patient. Interact Cardiovasc Thorac Surg. 2012; 14: 125-127.
- Marasco SF, Preovolos A, Lim K, Salamonsen RF. Thoracotomy in adults while on ECMO is associated with uncontrollable bleeding. Perfusion. 2007; 22: 23-26.
- Wen PH, Chan WH, Chen YC, Chen YL, Chan CP, Lin PY. Non-heparinized ECMO serves a rescue method in a multi-trauma patient combining pulmonary contusion and non-operative internal bleeding: a case report and literature review. World J Emerg Surg. 2015; 10: 15.
- Cronin B, Maus T, Pretorius V, Nguyen L, Johnson D, Ovando J, et al. Case 13--2014: Management of pulmonary hemorrhage after pulmonary endarterectomy with venovenous extracorporeal membrane oxygenation without systemic anticoagulation. J Cardiothorac Vasc Anesth. 2014; 28: 1667-1676.