
Case Report
Austin J Pulm Respir Med 2015; 2(3): 1033.
Different Performance and Prognosis for a Familial Case of Sarcoidosis in China: Novel Insight from Clinical Practice
Lei Liu¹* and Bin Liu²
¹Department of Respiratory Medicine, The Fourth Affiliated Hospital of Harbin Medical University, China
²Department of Respiratory Medicine, The First Affiliated Hospital of Harbin Medical University, China
*Corresponding author: Lei Liu, Department of Respiratory Medicine, The Fourth Affiliated Hospital of Harbin Medical University, No.37, Yi Yuan Street, Nan Gang District, Harbin 150001, China
Received: October 10, 2015; Accepted: November 17, 2015; Published: November 19, 2015
Abstract
Sarcoidosis is a common multi-system disease characterized histolopathologically by the formation of non-caseating granulomas in the affected tissue. Hereditary susceptibility to sarcoidosis is suggested by ethnic preponderance, familial clustering, and multigenerational involvement, but the genetics of sarcoidosis do not be adequately confirmed, so do the living environment, especially in china. This report presents a 55-year-old woman with blood-tinged sputum and exertional dyspnea. The clinical, laboratory examination and pathology revealed that the patient had sarcoidosis (stage II). Appropriate therapy was administered, and the patient symptom and sign were improved significantly. Her son was a patient with pulmonary sarcoidosis (stage II) contemporarily, however, no symptom and sign were revealed and self-healing happened. Her mother was a patient with pulmonary fibrosis which caused by sarcoidosis in family history, although appropriate therapy was given, but the patient symptom and sign were not improved significantly, her mother died because of serious lung fibrosis and combined infection. Nothing living environment and infection factors were found. There were different performance and prognosis for a Chinese family case of genetic predisposition sarcoidosis and the same disease remain needs individualized treatment.
Keywords: Sarcoidosis; Non-caseating granulomas; Individualized treatment
Introduction
Sarcoidosis is a common multi-granulomatous disease that is characterized by the formation of non-caseating epithelioid cell granulomas in various tissues and organs. The first case of sarcoidosis described in literature was by Hutchinson in 1875 year [1]. The sarcoidosis mainly affects the lungs, lymph nodes, liver, and spleen, and less frequently the eyes, bones and skin [1-3]. Sarcoidosis have a tendency of genetic disease, in the USA, there is significant racial variation in the incidence rate, with 10-14 cases per 100,000 for caucasians and 35.5-64 cases per 100,000 for African-Americans [4], the familial case report becomes a very rare finding in china, furthermore, the etiology has not been confirmed. There is a slightly higher incidence in females [4].
Case Report
We report the Chinese case of a 55-year-old (60 Kg) woman who had had chronic productive cough with blood-tinged sputum and exertional dyspnea for one year. She was a lifelong non-smoker, currently unemployed, and occasionally homeless. Nothing to be declared in her past medical history. Her son (30-year-old) was a patient with pulmonary sarcoidosis (stage II) contemporarily, however, no symptom and sign were revealed in her son, sarcoidosis was discovered by chest Computed Tomography (CT) and confirmed by bronchoscopic with biopsy tissue pathology 6 months, and selfhealing happened by chest CT in her son 3 months ago.
On examination, she was ill-appearing but in no acute distress. Her axillary temperature was 36.5oC; blood pressure was 130/80mm Hg with a normal heart rate of 80 bpm, and oxygen saturation SpO2: 95%. Lung auscultation revealed crackles over the lower right lung.
On laboratory testing, the patient had a normal level white blood cell count of 8,300 cells/uL; the hemoglobin level was 133 g/L. The erythrocyte sedimentation rate was 25 mm after the first hour. Hepatitis virus was negative. Nothing was abnormal in liver and renal function. Nothing was found about autoantibody examination and rheumatic test.
There was no lymphadenopathy observed in general examination, but chest CT demonstrated right hilar prominence, and hilar lymphadenopathy, and bilateral lower lung lobe infiltration opacification (Figure 1). In addition, bronchoscopic biopsy was conducted as a diagnostic approach.
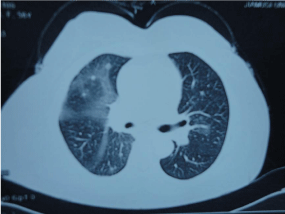
Figure 1: Computed tomography scan of the chest, right hilar prominence, and
hilar lymphadenopathy, and bilateral lower lung lobe infiltration opacification.
Pulmonary function testing after remission of the productive cough (one day) showed a restrictive pattern with a Forced Vital Capacity (FVC) of 54% predicted, a Forced Expiratory Volume in one second (FEV1) of 56% predicted, and an FEV1 /FVC ratio of 86%. The total lung capacity was 79% of predicted. There was reduction of the diffusion lung capacity for carbon monoxide to 56% of predicted.
Fiberoptic bronchoscopy revealed a partial extraluminal compression of the trachea and irregular distribution (scattered and beaded) of nodules in lobe and segmental brochial walls (Figure 2). No evidence of malignancy, vasculitis, or alveolar wall necrosis was found on biopsy, except tracheal mucosa chronic inflammation and non-caseating epitheloid cell granulomas formation with biopsy tissue pathology (Figure 3). The cytological results from the Bronchoalveolar Lavage Fluid (BALF) showed no malignancy. The T4/T8 ratio on the BALF was 1.9, while the blood T4/T8 ratio was 1.8. Microbiology cultures of the BALF were nothing. The smear for acid-fast bacilli was negative, and cultures for mycobacteria remained negative after 6 weeks.
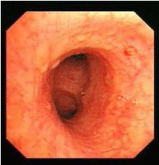
Figure 2: Beaded distribution of nodules in right lower lobe brochial wall by
fiberoptic bronchoscopy.
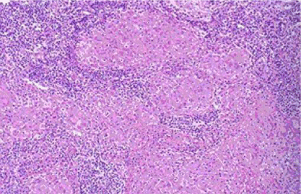
Figure 3: Tracheal mucosa chronic inflammation and non-caseating epitheloid
cell granulomas formation with biopsy tissue pathology. Hematocylin and
eosin stain, original magnification ×200.
According to Miller A et al. [5], diagnosis of stage II of sarcoidosis was suggested, she was informed and gave a written consent for side effect of the prednisolone, she was treated with prednisolone (40 mg/ day). Follow-up chest CT also showed normal pulmonary hilum, and remission of irregular distribution of nodules and bilateral lower lung lobe infiltration opacification after treatment of 2 weeks, and disappearing symptom and crackles over the lower right lung was found by examination as well (Figure 4). Further follow-up is going on, and gene detection may be done next.
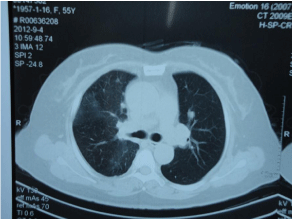
Figure 4: Computed tomography scan after treatment of 2 weeks follow-up.
Discussion
The etiology of sarcoidosis is not well understood. There is a genetic predisposition suggested by the presence of familial clusters in sarcoidosis [6]. There are no specific tests for sarcoidosis, diagnosis of sarcoidosis is mainly based on clinical characteristics [1,3], especially in china, as well as chest imaging examination and the typical non-caseating granulomas which contain epithelioid macrophages surrounded by lymphocytes, exclusion of other noncaseating granulomas forming conditions.
In sarcoidosis immunopathogenesis, the immune system undergoes a reactivity change. Primary features are a reduction in circulation CD4+ lymphocytes, while their numbers in tissue increase, which is associated with a remarked increase in tissue cytokine production, particularly interferon-γ, interleukin-2, and so on [7]. The T-helper 1 cytokine profile recruits macrophages, eventually forming a granulomatous reaction [8]. The production of inhibitory cytokines by macrophages appears to interfere with the normal immune response. Girard et al. reported five cases and reviewed 65 additional cases of opportunistic infections associated with sarcoidosis [9]. Observations have suggested a pathogenetic role of Gram-negative infections in sarcoidosis by driving and interleukin-18 response as another feature of the Th-1 pathway [10]. Nothing living environment and infection factors were found in the case, it demonstrates that sarcoidosis may, in rare cases, report as a tendency of genetic disease in Chinese. There were different performance for this case of genetic sarcoidosis, the patient need be treated and the son was self-healing. Her mother was a patient with sarcoidosis, too, in her family history, although appropriate therapy similar to herself therapy was administered, but the patient symptom and sign were not improved significantly, pulmonary fibrosis happened; her mother died because of serious lung fibrosis and combined infection. There were different performance and prognosis for a Chinese family case of genetic predisposition sarcoidosis, the same disease remains needs individualized treatment. Inquiry, examination, and inspection are needed, the more detail date, the more individualized treatment, and the better prognosis may happen. Gene detection may be needed in future.
Consent
The patients signed consent and gave permission to use the photos in the manuscript.
References
- Suresh L, Radfar L. Oral sarcoidosis: a review of literature. Oral Dis. 2005; 11: 138-145.
- Kasamatsu A, Kanazawa H, Watanabe T, Matsuzaki O. Oral sarcoidosis: report of a case and review of literature. J Oral Maxillofac Surg. 2007; 65: 1256-1259.
- Marie I, Proux A, Levesque H, Bony-Rerolle S, Chenal P. Tongue involvement revealing sarcoidosis. QJM. 2008; 101: 909-911.
- Thomeer M, Demedts M, Wuyts W. Epidemiology of sarcoidosis. Drent M, Costabel U, editors. In: Sarcoidosis. 1st Edn. Wakefield: European Respiratory Monograph. 2005: 13-22.
- Miller A, Chuang M, Teirstein AS, Siltzbach LE. Pulmonary function in stage I and II pulmonary sarcoidosis. Ann N Y Acad Sci. 1976; 278: 292-300.
- Rybicki BA, Harrington D, Major M, Simoff M, Popovich J Jr, Maliarik M, et al. Heterogeneity of familial risk in sarcoidosis. Genet Epidemiol. 1996; 13: 23-33.
- Möllers M, Aries SP, Drömann D, Mascher B, Braun J, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax. 2001; 56: 487-493.
- Agostini C, Facco M, Chilosi M, Semenzato G. Alveolar macrophage-T cell interactions during Th1-type sarcoid inflammation. Microsc Res Tech. 2001; 53: 278-287.
- Girard N, Cottin V, Hot A, Etienne-Mastroianni B, Chidiac C, Cordier JF. [Opportunistic infections and sarcoidosis]. Rev Mal Respir. 2004; 21: 1083-1090.
- Antoniou KM, Tzouvelekis A, Alexandrakis MG, Tsiligianni I, Tzanakis N, Sfiridaki K, et al. Upregulation of Th1 cytokine profile (IL-12, IL-18) in bronchoalveolar lavage fluid in patients with pulmonary sarcoidosis. J Interferon Cytokine Res. 2006; 26: 400-405.