
Research Article
Austin J Pulm Respir Med 2016; 3(1): 1038.
Evaluation of Pediatric Empyema at Children’s Hospital from 2003 to 2013
Jeffers KL¹, Varman M²*, Noronha L³ and Wichman C4
¹Creighton University School of Medicine, USA
²Pediatric Infectious Diseases, Creighton University School of Medicine, USA
³Pulmonary and Critical Care Medicine, Children’s Hospital & Medical Center, USA
4Biostatistics, Research and Compliance Office, Creighton University, USA
*Corresponding author: Varman M, Pediatric Infectious Disease, Creighton University School of Medicine, 601 N 30th Street, Suite 6814, Omaha, Nebraska 68131, USA
Received: February 29, 2016; Accepted: March 25, 2016; Published: March 28, 2016
Abstract
The diagnosis and treatment of pediatric empyema can vary greatly. Diagnosis, etiology, and treatment of empyema, and their effects on hospital Length of Stay (LOS) were investigated.
We analyzed 136 children. Mean LOS was 14.99 days. For imaging, 8% (10/131) had a chest ultrasound, 60% a CT scan (79/131), 14% (18/131) a chest x-ray and 18% (24/131) had both CT scan and ultrasound. For intervention 18% (24/136) received only antibiotics, 50% (69/136) a chest tube, 25% (33/136) a Video Assisted Thoracoscopic Surgery (VATS), and 7% (10/136) an open procedure. Size of effusion had no impact on intervention type or LOS. Etiology was identified in 49% (66/136). 9% (12/136) had MRSA, 19% (26/136) had Streptococcus pneumoniae, 12% (17/136) had Group A Beta Hemolytic Streptococci (GABHS), and 8% (11/136) had others.
Children age 2 to 12 and those receiving antibiotics only had a shorter LOS. Empyema from “other’ organisms’ category had a longer LOS. The highest mean white blood cell count (31.71 thousand/cmm) was in GABHS and the highest CRP in Streptococcus pneumoniae (24.96 mg/dl).
Ultrasound was less frequently used than CT scan. A shorter LOS was seen when antibiotics alone were used. Organisms in the “other’ category had a longer LOS.
Keywords: Pediatric empyemal; Diagnosis; Treatment; Chest ultrasound; Computed tomography; Antibiotics
Abbreviations
CRP: C Reactive Protein; GABHS: Group A Beta Hemolytic Streptococci; LOS: Length of Stay; MRSA: Methicillin Resistant Staph aureus; VATS: Video Assisted Thoracoscopic Surgery
Introduction
Empyema is defined as a purulent pleural fluid collection in association with an underlying pneumonia [1-3]. Classically the patient presents with symptoms of pneumoniae with persistent fever, malaise, and lethargy while a cough and tachypnea develop as the disease progresses [1,4]. An initial blood count may show leukocytosis, thrombocytosis, and anemia but these are non-specific markers [1]. A chest x-ray is recommended on all patients with signs of a pleural effusion, but chest ultrasound is the gold standard to determine the location of the fluid accumulation, its extent and stage, and can be used to guide drain insertion [5,6]. Even though CT scan of the chest may provide additional information it should rarely be used, as it does not alter treatment [1,3].
Rapid diagnosis is important, as a delay in treatment has been shown in adults to have a significant increase in morbidity. The bacterial causes of empyema vary by region, but Streptococcus pneumonia is the most common in developed countries. Other causative organisms are GABHS, Staphylococcus aureus, and Haemophilus influenza [1-3,7-9].
Although the management of empyema varies by institution, overall outcomes in children are good, compared to that in adults [1]. Current options for management are antibiotics alone and antibiotics combined with a percutaneous chest drain. Fibrinolytics can be used and have been shown to be effective about 80-90% of the time for loculated empyema, but less research has been done on their use [1-4,10]. When medical therapy fails in advanced cases of empyema, surgical intervention in addition to antibiotics is required for management. The surgical options are 1. Open thoracotomy, a complicated procedure but it has been shown to be associated with shorter hospital stays. 2. Mini-thoracotomy is similar but involves a smaller incision while still being an open procedure. 3. Video Assisted Thoracoscopic Surgery (VATS) is less invasive, but requires physicians and facilities capable of the procedure [10]. VATS has also been associated with a shorter hospital stay [1]. Overall resolution depends on adequate drainage of the infected material from the pleural space in addition to appropriate antibiotic therapy.
Currently many aspects of empyema management from diagnosis to treatment vary by physician and institution. Few studies have been done regarding the optimal management of empyema, and even fewer in the pediatric population. This study reviews the diagnostic evaluation of empyema, particularly looking at utilization of ultrasound compared to chest CT scan for diagnosis. We assessed the factors impacting Length of Stay (LOS). The outcomes of this study may be applicable as quality improvement measures at Children’s Hospital.
Methods
In this retrospective study we reviewed the clinical and microbiological records of all children, birth to 18 years old, with a diagnosis of empyema from Jan 2003-Dec 2012. After IRB approval we collected the data from Children’s Hospital in Omaha, Nebraska, a community hospital. Exclusion criteria included patients with malignancy to prevent inclusion of malignant effusions or cardiac surgery as their primary procedure to prevent inclusion of effusions secondary to a cardiac cause.
Data collected included diagnostic method, size of effusion based on radiologists report, etiology, peripheral white blood count, C Reactive Protein (CRP), and interventions that were performed. We classified the patients as having had only antibiotics, having a chest tube and antibiotics, any combination of treatment that led to VATS and any combination of treatment that led to an open procedure.
Statistics
A multivariate analysis of the data was performed using both a forward and backward multiple logistic regression analysis with a level of significance set at p< 0.05.
Results
We analyzed a total of 140 charts, 136 were included for analysis, 67 females and 69 males, with empyema from Jan 2003 to Dec 2012. Four children were excluded. One with a primary admission for open-heart surgery, two that were readmissions related to a previous empyema and one for lack of records. For diagnostic imaging, 8% (10/131) had an ultrasound 60% (79/131) a CT scan, 14 % (18/131) had only a chest x-ray and 18% (24/131) had both CT scan and ultrasound. The number of each diagnostic method performed per year can be seen in Figure 1A.
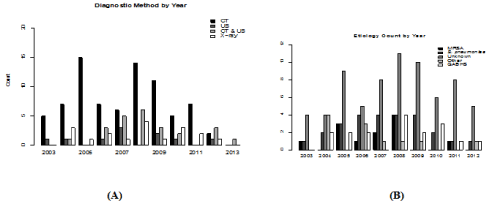
Figure 1: (A) Use of different diagnostic methods each year of the study. (B) Causative organisms of empyema identified by year.
For intervention 18% (24/136) of patients received only antibiotics, 50% (69/136) chest tube placement, 25% (33/136) VATS, and 7% (10/136) an open thoracotomy procedure in addition to antibiotics. Mean LOS for the study was 15 days. The antibiotic group had a mean LOS of 10 days, the chest tube group 15.5 days, the VATS group 17.5 days, and the open procedure group stayed for an average of 20 days. Confidence intervals for the true mean for each factor affecting LOS can be seen in Figure 2. Size of effusion was a nonconfounding variable and had no impact on type of intervention or ultimate LOS.
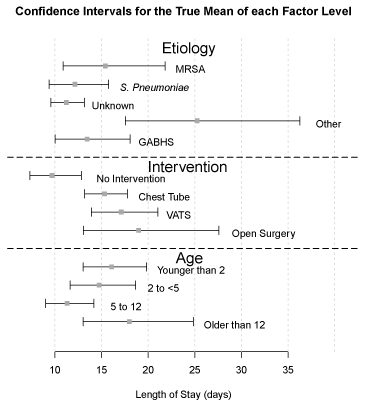
Figure 2: Confidence interval for the true mean of each factor level: etiology,
intervention and age.
We identified the etiology in 48.5% (66/136) of the patients with empyema. Of these 9% (12/136) had Methicillin Resistant Staphylococcus aureus (MRSA), 19% (26/136) Streptococcus pneumoniae, 12% (17/136) GABHS, 8% (11/136) others (fusobacterium, pseudomonas, Streptococcus viridans, enterobacter, Methicillin Susceptible Staphylococcus aureus (MSSA), and prevotella), and 51% (70/136) unknown etiology. Etiology diagnosed by year can be seen in Figure 1B.
Of the variables studied, several had an impact on LOS. Children between the ages 2 years and 12 years and those receiving antibiotics only had a shorter LOS. Empyema due to “other’ category of organisms had a longer LOS. We noted the highest mean White Blood Cell count (WBC) of 31.71 thousand/cmm in GABHS whereas Streptococcus pneumoniae had the highest mean CRP of 24.96 mg/dl. The means for both CRP and WBC can be seen in Figure 3.
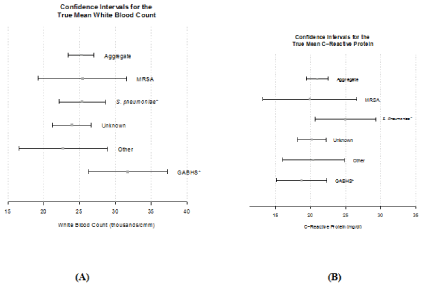
Figure 3: (A) Confidence interval for the true mean WBC. (B) Confidence
interval for the true mean CRP.
Discussion
One of the goals of the study had been to assess the utilization of ultrasound at Children’s Hospital as it is currently considered the gold standard for diagnosis [1,5,8]. We hypothesized there would be an increase in the number of ultrasounds done compared to CT scan of the chest over the course of the study. There was an upward trend in the number of ultrasounds for diagnosis, but the use of ultrasound alone was infrequently done as shown in Figure 1A. Often a patient received both an ultrasound and subsequently a CT scan. Ultrasound is cost-effective as opposed to CT, and has no radiation risks in diagnosis of empyema. CT, while it may provide additional information, that information often does not alter the course of treatment, and is unable to predict clinical outcomes [1,3]. But CT scan, due to the finer details it provides, is invariably performed prior to surgical intervention.
Another factor analyzed was size of effusion. The size of effusion, and likely severity of disease, had no impact on type of intervention, or the ultimate length of stay. This is consistent with the findings in Goldin’s study of pediatric empyema [10]. He found that many providers believe treatment algorithms for empyema should be stratified according to severity of disease meaning children with a more severe illness would be more likely to receive and benefit from an invasive procedure. Consistent with previous research we found that size of effusion had no impact on the length of stay or the interventions chosen.
Goldin et al. showed that almost half of the children received only antibiotics with good outcomes, and which is associated with the greatest decrease in mean LOS while in the literature, VATS has been shown to have a shorter LOS [2,10]. Our data also showed that children treated with antibiotics alone had a statistically significant shorter LOS. Although our study did not show a statistical difference in LOS with the use of VATS, children who received a VATS as compared to an open procedure had an average of 2.5 days shorter LOS. We also found that children less than 2 years and older than 12 years had a longer average LOS. Children in middle age ranges seem to tolerate the disease better than the very young or adolescents as can be seen in the confidence intervals for the true mean in Figure 2.
As is commonly seen in other studies, and our data, a causative organism is identified about 50% of the time [2]. The most common organism cultured in the developing world is Streptococcus pneumonia, which was also the organism most frequently identified in our study [1,2,4,9]. All organisms identified each year can be seen in Figure 1B. Of interest, we noted an increased LOS when organisms in the other category caused the empyema. The other category of organisms included fusobacterium, pseudomonas, streptococcus viridans, Enterobacter, MSSA, and prevotella. Several of these organisms are common to aspiration pneumonia and about half of the children with empyema from this category had a developmental delay or physical handicap placing them at higher risk for aspiration. It is possible that their comorbidities also added to the overall increased length of stay for empyema related to this group of organisms.
It is also of interest to look at the incidence of Streptococcus pneumonia throughout the study in regards to the possible effects of the introduction of the Prevnar 13 vaccine. The first conjugate pneumococcal vaccine was licensed in 2000 in the United States, and is recommended to be given during the first six months of life, but can be extended out up to 5 years of age [11]. Large-scale studies have proven the effectiveness at reducing incidence of invasive pneumococcal disease. In our own study, there was no significant decrease in the number of cases of empyema due to Streptococcus pneumonia over the course of the study, which could be due to unknown etiology in our study.
CRP and WBC count were the other variables we assessed in the study. We found the highest WBC in cases where empyema was attributed to GABHS while the greatest increase in CRP was due to Streptococcus pneumoniae. The confidence intervals for the true mean for these variables can be seen in Figure 3A and 3B. Of the studies done on pediatric empyema; few have looked at the etiologic organisms and their effect on inflammatory markers. One study looked at Red Cell Distribution Width (RDW) and found that it was a predictor of mortality, but no other work has been done to corroborate it. More work should be done on this in the future.
Conclusion
Our study identified that 1) chest ultrasound is being used less frequently than chest CT scan for diagnosis of empyema, 2) a shorter LOS occurs when antibiotics alone were used compared to any invasive intervention in combination with antibiotics and 3) organisms in the other category, predominantly among children with neurodevelopmental concerns, had a longer LOS likely related to aspiration. More work needs to be done on better laboratory studies for the identification of etiologic organisms, the effects of different etiologies on inflammatory markers and disease processes, and deciding when best to use an invasive method and the most appropriate procedure to use.
References
- Yousef A, Jaffe A. The management of pediatric empyema. Hong Kong Journal of Paediatrics. 2009; 14: 16-21.
- Marks DJ, Fisk MD, Koo CY, Pavlou M, Peck L, Lee SF, et al. Thoracic empyema: a 12-year study from a UK tertiary cardiothoracic referral centre. PLoS One. 2012; 7: e30074.
- Hawkins JA, Scaife ES, Hillman ND, Feola GP. Current treatment of pediatric empyema. Semin Thorac Cardiovasc Surg. 2004; 16: 196-200.
- Le Monnier A, Carbonnelle E, Zahar JR, Le Bourgeois M, Abachin E, Quesne G, et al. Microbiological diagnosis of empyema in children: comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin Infect Dis. 2006; 42: 1135-1140.
- Sandweiss DA, Hanson JC, Gosink BB, Moser KM. Ultrasound in diagnosis, localization, and treatment of loculated pleural empyema. Ann Intern Med. 1975; 82: 50-53.
- Mahal AS, Weisse AB, Bolanowski PA, Reichman LB. Letter: Ultrasound in diagnosis of empyema. Ann Intern Med. 1975; 83: 123-124.
- McLaughlin FJ, Goldmann DA, Rosenbaum DM, Harris GB, Schuster SR, Strieder DJ. Empyema in children: clinical course and long-term follow-up. Pediatrics. 1984; 73: 587-593.
- Brook I. Microbiology of empyema in children and adolescents. Pediatrics. 1990; 85: 722-726.
- Obando I, Muñoz-Almagro C, Arroyo LA, Tarrago D, Sanchez-Tatay D, Moreno-Perez D, et al. Pediatric parapneumonic empyema, Spain. Emerg Infect Dis. 2008; 14: 1390-1397.
- Goldin AB, Parimi C, LaRiviere C, Garrison MM, Larison CL, Sawin RS. Outcomes associated with type of intervention and timing in complex pediatric empyema. Am J Surg. 2012; 203: 665-673.
- Pneumococcal Disease. Center for Disease Control and Prevention. CDC. Web. 2015.