
Research Article
Austin J Pulm Respir Med 2016; 3(2): 1045.
Pleural Sonogram: Tissue Attributes and Guide for Forceps Biopsy
Abumossalam AM¹*, Abdalla DA¹, Abd E¹_khalek AM² and Shebl AM³
¹Thoracic Medicine Department Mansoura University, Egypt
²Radiodiagnosis Department, Mansoura University, Egypt
³Pathology Department, Mansoura University, Egypt
*Corresponding author: Ahmed Mohammed Abumossalam, Mansoura University Hospital, Thoracic Medicine Department, Mansoura Gomheria Street 35516, Egypt
Received: August 04, 2016; Accepted: October 03, 2016; Published: October 05, 2016
Abstract
Methods: In our study forty two patients with exudative pleural effusion included in this study. They underwent TTU using Philips HD 5; superficial two dimensional probe 7-11 MHz for assessment of echographic features of pleural based lesions and observing their linkage with the final diagnosis carried out by ultrasound guided forceps biopsy by using Douay cervical biopsy forceps - 20 cm), trocar and cannula of Anderson needle and closing the inlet by rubber seal.
Results: Neoplastic disorders were arranged as metastatic adenocarcinoma by 57.1% followed by malignant lymphoma and malignant mesothelioma (each by 9.5%) and lastly squamous cell carcinoma (4.8%). Regarding non-neoplastic lesions; tuberculous pleurisy accounted for 7.1% while nonspecific pleurisy showed 11.9%. All echogenic parameters showed highly significant statistical differences among types of pleural lesions (p < 0.001).
Keywords: Forceps Biopsy- Pleural Ultrasound – Malignant- Sonographic features - Tubercuolous
Abbreviations
TTU: Transthoracic Ultrasound; MHz: Mega Hertz; HD: High Definition; US: Ultrasound; cm: centimeter; ml: milliliter; mg: milligram; SPSS: Statistical Package for Social Sciences; ANOVA: Analysis of Variance
Introduction
Ultrasonography (US) is a long-established complementary imaging modality in the diagnosis of pleural effusions. Technical development and ongoing scientific evidence have expanded the spectrum of application for sonography in diseases of the chest over the last few years [1]. With the proper examination method, the whole costal and diaphragmatic pleura can be visualized. According to estimates based on computed tomography, at least 60–70% of the pleura surface can be visualized sonographically [2]. The costal and diaphragmatic pleural segments are the frequent site for most of pleura diseases [3]. Chest sonography has been found useful in detecting pleural and pleural based lesions [4], and evaluating pleural involvement by lung tumor [5] as well as, defining localizing loculated or minimal effusion before thoracentesis [6-8]. The value of color duplex sonography of the pleura, however, has not been systematically evaluated, but it is helpful in distinguishing tumor-like lesions and infiltrations. Color duplex sonography, spectral Doppler sonography and contrast-enhanced sonography have achieved a position of significance in the differential diagnosis of space-occupying lesions at the level of the pleura [3]. Thoracoscopy and thoracotomy are the ultimate choice for diagnosing pleural effusion owing to feasibility to take biopsy from multiple pleural sites under vision. However, these latter procedures are associated with certain precautions, complications and discomfort to the patient [9,10]. US guidance increases the diagnostic success rate and decreases the complications associated with interventional procedures such as thoracocentesis, closed tube drainage for pleural effusion and needle biopsy of the pleura [11]. Moreover, it provides adequate tissue sampling of lesions for cytological, histological or microbiological analysis [12]. One of the image-guided procedures is forceps biopsy of the pleura under sonographic guidance which enable the physician to take biopsy from multiple pleural sites and can meet the shortfall between the needle biopsy and those invasive procedures [13,14]. So this work aimed to assess the value of TTU to diagnose pleural based lesions based on sonomorphological criteria and as guidance for forceps tissue biopsy as an alternative method for conventional blind pleural biopsy.
Patients and Methods
A prospective interventional simple double blinded randomized controlled trial (first clinical trial in Mansoura University Thoracic Medicine Department) was conducted on forty two patients with exudative pleural effusion based on Light criteria, 2011 [15]. They were assembled from out-patient clinic of Thoracic Medicine department, Mansoura University Hospitals during the period from July 2014 to December 2015. Patients signed their written consents after detailed explanation of the study protocol. Local ethical approval had been obtained.
Inclusion criteria: Patients with exudative pleural effusion
Exclusion criteria:
1- Previously known etiology of pleural effusion.
2- Transudative pleural effusion.
3- Frozen chest due to organized empyema or pleurodesis.
4- Patients with bleeding tendency or blood coagulation defects.
5- Obese patients
6- Severely disabled patients
7- Patient with skeletal deformities and kyphoscoliosis.
Procedure
Pleural echography: - The patients were evaluated with the following echographic signs during the TTU procedure using ultrasound apparatus (Philips HD 5 , Japan, with superficial two dimensional probe 7-11MHz) ; pleural sonomorphology
1- Shape of pleural based lesion.
2- Pleural layer involved.
3- Echogenecity
4- Secondary changes (necrosis and degenerations)
5- Doppler study (decreased vascularity or increased with neovasculatrization).
6- Echoinvasion of surrounding structures.
7- Borders of the pleural lesion (ill defined / well defined) [16].
Forceps biopsy: - Patients were submitted for the procedure once. The study was conducted via an ultrasound apparatus (Philips HD 5, Japan, with superficial two dimensional probe 7-11MHz), biopsy forceps (Douay cervical biopsy forceps - 20 cm), trocar and cannula of Anderson needle and closing the inlet by rubber seal. The Procedure was carried out using the two-hand technique under US observation. The desired skin site for instrument insertion was determined according to the site of the pleural lesion which was identified by US. The patient was then received analgesic (biprofenid 150 mg) in a sitting position. The skin at the biopsy site was sterilized and anesthetized with 5–10 ml of 2% xylocaine followed by making a stab incision with size 11 scalpel blade alongside the intended biopsy track. The skin incision was followed by introduction of the trocar and cannula into the pleural space. The trocar was then withdrawn and the mouth of the cannula occluded with the finger pressure simultaneously with closure of the cannula valve to prevent leaking of any air into the pleural cavity. Rubber inlet seal was then fixed at the mouth of the cannula to ensure that no fluid or air could pass during introduction of the forceps. During forceps introduction through the cannula; the valve was opened and simultaneously the US probe was applied to the chest wall enveloped in sterile gloves with enclosed gel. The operator held the cannula and the biopsy forceps while the assistant held the US probe in order to direct the operator to the pleural lesion to take biopsy. Following biopsy, the forceps withdrawn gradually and the cannula valve closed. Further biopsy from different sites was achieved by the reintroduction of the forceps and changing the angle of the cannula at the skin simultaneously with the changing position of the probe. The incision was then sutured by 2 zero stitches silk. All biopsies were placed in 10% formalin and sent to the pathologist for histopathological examination. Medical thoracoscopy was done for cases with nonspecific pleurisy that confirmed the diagnosis of chronic fibrinous pleurisy. Assurance of the results exceeded those procedures by following up these patients either with neoplastic and nonneoplastic disorders.
Statistical analysis
Data was analyzed using SPSS (Statistical Package for Social Sciences) version 21. Qualitative data was presented as number and percentage. Quantitative data was presented for normality by Kolmogrov-Smirnov test. Normally distributed data was presented as mean and standard deviation. Comparison between groups was done using one way ANOVA test. Student t-test was used to compare between two groups. P value < 0.05 was considered significant.
Results
Table 1 showed that this study was conducted on forty two patients with mean age of studied cases was 53.71 with standard deviation 18.072 with median 53. Their sex was matched (50% male and 50% females) with higher median age in metastatic adenocarcinoma and nonspecific pleurisy than other pathological pleural lesions.
Age of studied cases
Mean ± SD
53.71 ± 18.072
Median
53.00
Final Diagnosis
Malignant lymphoma
No=4
Malignant mesothelioma
No=4
Metastatic adenocarcinoma
No=24
Squamous cell carcinoma
No=2
Non-Specific pleurisy
No=5
TB pleurisy
No=3
Total
No=42
Age Median
in years
40
38
63
50
63
50
Female
2
1
12
2
1
3
21 (50%)
Male
2
3
12
0
4
0
21 (50%)
Total
4
4
24
2
5
3
42
P value
(sex difference)
0.078
Table 1: Age and sex distributions in studied cases.
Table 2 and Figure 1 presents the final outcome of the US guided forceps biopsy. Neoplastic disorders were arranged as metastatic adenocarcinoma by 57.1% followed by malignant lymphoma and malignant mesothelioma (each by 9.5%) and lastly squamous cell carcinoma (4.8%). Regarding nonneoplastic lesions; tuberculous pleurisy accounted for 7.1% while nonspecific pleurisy showed 11.9%.
Biopsy Results
Frequency
Percent
Malignant lymphoma
4
9.5
Malignant mesothelioma
4
9.5
Metastatic adenocarcinoma
24
57.1
Squamous cell carcinoma
2
4.8
TB pleurisy
3
7.1
Non-Specific pleurisy
5
11.9
Total
42
100
Table 2: Final diagnosis of ultrasound guided forceps biopsy in studied cases.
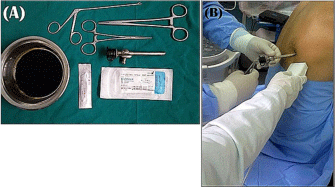
Figure 1: Illustration of the instruments used in biopsy sampling and
ultrasound guidance during the maneuver.
(A)- Biopsy forceps (Douay cervical biopsy forceps - 20 cm), trocar and
cannula of Anderson needle and closing the inlet by rubber seal.
(B)- Ultrasound guidance with two hand method.
Table 3 gives an idea about the sonomorphological parameters in the different pathological outcomes. As regards neoplastic lesions; malignant lymphoma showed predominant characterization in visceral and parietal pleural involvement, presence of secondary changes with echoinvasion, and ill-definition of the borders (100%). Hyperechogenecity and decreased vascularity were detected in 75% of cases but the masses and plaques showed equal percentage. Regarding malignant mesothelioma; masses, visceral and parietal pleural affection, increased vascularity, hypoechogenecity were noticed in 75% of cases while all cases showed secondary changes with echoinvasion and ill-definition of the borders. Metastatic adenocarcinoma showed an evidence of prevalence of nodules, echoinvasion, illdefinition of borders, hyperechogenecity with increased vascularity (70.8%, 87.5%, 87.5%, 100% and 100% respectively). Squamous cell carcinoma predominated in masses with visceral affections, secondary changes with echoinvasion plus ill-definition of borders, and lastly hpoechogenecity with increased vascularity. Nevertheless, non neoplastic lesions in the form of nonspecific pleurisy prevailed in parietal pleural thickenings as well as hyperechogenecity with decreased vascularity. On the other hand, in tuberculous pleurisy; two thirds of cases evidenced parietal and visceral pleural thickenings besides hyperechogenecity with decreased vascularity. Echoinvasion, ill-definition of borders and secondary changes were lacking in nonneoplastic lesions. All echogenic parameters showed significant statistical differences among types of pleural lesions (p value< 0.001).
Malignant lymphoma
N0=4
Malignant mesothelioma
N0=4
Metastatic adenocarcinoma
N0=24
Squamous cell carcinoma
N0=2
Non-Specific pleurisy
N0=5
TB pleurisy
N0=3
Total
42
Shape of lesion
Masses
2 (50%)
3 (75%)
7 (29.2%)
2 (100%)
0
0
14
Nodules
0
0
17 (70.8%)
0
0
0
17
Plaques
2 (50%)
0
0
0
0
1 (33.3%)
3
Rind
0
1 (25%)
0
0
0
0
1
Thickening
0
0
0
0
5 (100%)
2 (66.7%)
7
P value
0.000
Pleura involved
Parietal
0
0
0
0
4 (80%)
0
4
Visceral
0
1 (25%)
13 (54.2%)
2 (100%)
1 (20%)
1 (33.3%)
18
Both
4 (100%)
3 (75%)
11 (45.8%)
0
0
2 (66.7%)
20
P value
0.000
Secondary changes
4 (100%)
4 (100%)
6 (25%)
2 (100%)
0
0
16
P value
0.000
Echoinvasion
4 (100%)
4 (100%)
21 (87.5%)
2 (100%)
0
0
31
P value
0.000
Il-definition of lesion borders
4 (100%)
4 (100%)
21 (87.5%)
2 (100%)
0
0
31
P value
0.000
Doppler study
Increased vascularity
1 (25%)
3 (75%)
24 (100%)
2 (100%)
0
0
Decreased vascularity
3 (75%)
1 (25%)
0
0
5 (100%)
3 (100%)
P value
0.000
Echogenecity
Hypoechogenecity
1 (25%)
3 (75%)
24 (100%)
2 (100%)
0
0
Hyperechogenecity
3 (75%)
1 (25%)
0
0
5 (100%)
3 (100%)
P value
0.000
Table 3: Sonomorphological criteria of finally diagnosed pleural lesions in studied cases.
Table 4 demonstrated that minimal complications were encountered in our research (4.7%) in the form of pneumothorax in 2.35% and metastases in the biopsy tract in one case of malignant mesothelioma.
Complications
Number and percent
Pneumothorax
1 (2.35%)
Haemothorax
0
Broncho-pleural fistula
0
Spread of lesion
1 (2.35%)
Sinus tract
0
Death
0
Total
2 (4.7%)
Table 4: Complications of the ultrasound guided forceps biopsy in studied cases.
Discussion
In the later decades, with the purpose of managing pleural effusions, sonography turned to be used as the method of choice today. In fact, sonography is now used as a diagnostic modality to elucidate pleural effusions and also is a fixed element of guidelines concerned by pneumological societies [17]. The diagnosis can be established on the basis of effusion cytology or closed blind pleural biopsy (Rahmel needle, Abrams needle) in no more than 30% of patients. Biopsies taken by thoracoscopy allowed the diagnosis to be established in more than 90% of cases [18]. Percutaneous biopsies under sonographic guidance achieve nearly the same rate of accuracy [19].
Our end results in this research aspect underlined sonomorphological features of pleural lesions either benign or malignant and implications of sonographic guided forceps biopsy. A study by Bittner et al., 1995 stated that most of patients with non specific pleurisy have sonographically striking findings, generally hypoechogenically thickened parietal pleura, especially during the early stages of pleuritis. Besides the actual swellings of the pleura, echogenic fibrinous bands overlay the pleural layers in due course at a later stage of the pleuritis [20]. Color Doppler sonography of the expected hyperemia in pleurisy had, so far, proved to be rather substandard. Well vascularization could be observed in only 23.4% of cases and hence this method is limited in its application as a diagnostic signal [21]. Reuf and Schneider 1992 [22], concluded that pleural scars and fibrosis caused by putrid pleuritis appear variously echogenic and mainly hypocheoic on US. In later stages, scar may become more echogenic, sometimes with highly echogenic shadows indicative of calcifications. Those findings were matched with our work, benign pleural lesions in the form of nonspecific pleurisy presented by parietal pleural (80% of cases) thickenings (100% of cases) as well as hyperechogenecity with decreased vascularity (100% of cases). On the other hand in pleurisy caused by tuberculosis two thirds of cases evidenced parietal and visceral pleural thickenings above and beyond hyperechogenecity with decreased vascularity (100% of cases). Echoinvasion, ill-definition of borders and secondary changes were lacking in non-neoplastic lesions. That explained by the pathogenesis of fibrin deposition yielding tissue texture with minimal vascularity. Sonographic differentiation between benign and malignant pleural fluid is feasible only if solid nodular structures are visible [23,24]. Most metastases are found at the costal pleura or on the diaphragm, as well as in the angle between the diaphragm and the chest wall, even without an associated effusion. Pleural metastases are mainly hypoechogenic to moderately echogenic. They seem to be nodular, round-shaped to hemispherical or broad-based polypoid formations, which overhang prominently into the effusion [25]. Single, well delineated metastases cannot be distinguished from benign pleural tumors due to their similar sonomorphology [26].
Extensive or sheet-like infiltrations of the pleura in metastatic carcinomatosis with or without effusion are rarely seen [27]. In our research, there were conspicuous findings of metastatic adenocarcinoma that showed an evidence of prevalence of nodules, echoinvasion, ill-definition of borders, hypoechogenecity with increased vascularity (70.8%, 87.5%, 87.5%, 100% and 100% respectively). Squamous cell carcinoma exemplified in masses with visceral affections, secondary changes with echoinvasion plus illdefinition of borders, and lastly hpoechogenecity with increased vascularity.
Pleural mesothelioma was distinguished as calcified or noncalcified pleural thickening, typically occurring in the dorsolateral portions of the coastal region of the parietal pleura. Sonography, with its high local resolution, would be a constructive appliance to monitor the pleural changes of high-risk groups [28]. Approximately 10% of plaques become calcified, were then highly echogenic and caused visible shadows [29]. Mesotheliomas have very irregular, partly angular, unclear borders. In addition to tumor like formations, mesotheliomas can also present as extensive, tapestry like growths with nodules [2]. In our study, regarding malignant mesothelioma, masses were the predominant form besides increased vascularity, hypoechogenecity that noticed in 75% of cases while all cases showed secondary changes with echoinvasion and ill-definition oh the borders. No calcification was encountered in this lesion type in our work.
With reference to malignant lymphoma, it showed chief characterization in visceral and pleural involvement, presence of secondary changes with echoinvasion ill-definition of the borders and hyperechogenecity with decreased vascularity were detected in 75% of cases but the masses and nodules showed equal percentage (50% for each).
Echoinvasions of the chest wall and the diaphragm were visualized as striped, hypoechoic ramifications at the time of diagnosis [30]. Signs of infiltration include a faint, interrupted, or even absent delimitation of the metastasis from its surroundings, or a pseudopodium-like offshoot which, due to its lower echogenicity compared to the chest wall or the diaphragm, is often readily visible [26].
Our results went in the same behavior of other studies citing that all echogenic parameters showed significant statistical differences among types of pleural lesions (p value< 0.001), that ascertain eminent reliance upon them as trustee differentiating parameters.
Many studies revealed that even after several modifications of needle design and biopsy procedures; closed needle biopsy allows taking biopsy only from the costal part of the parietal pleura and cannot take biopsy from the diaphragmatic and mediastinal parts that commonly affected in malignant effusion [31-34].
Studies declared that biopsy of extra pleural tissues took place in 0.8% of cases during the needle biopsy more with minimal fluid (16%) [34,35]. Regarding the diagnostic yield, needle biopsy is usually positive in 40–60% of patients with malignant pleural disease while in patients with tuberculosis, it is positive for granuloma in 50–80% [36].
By US guided forceps biopsy; multiple bites from different sites inaccessible to the biopsy needle and parts of the parietal pleura could be attempted being simpler, less traumatic, more suitable and comfortable than thoracoscopy that requires sedation, analegesia ,chest tube drainage for lung expansion and is also associated with complications [37]. By using the US-guided forceps; it was possible to get the final pathological diagnosis in 11 out of 12 patients with pleural effusion as reported previously by Seitz et al. [14].
In study by Gamal Agmy et al., 2013 [38], US-guided forceps biopsy of the pleura helped them to reach final pathological diagnosis in 84 out of 96 patients with pleural effusion. The sensitivity of USguided forceps pleural biopsy in the diagnosis of malignant and tuberculous lesions was 85% and 88% respectively. In our study, fortunately, all cases done by forceps biopsy were diagnosed due to careful selection of cases, exclusion of obese disabled patients and those with inaccessible sonographic window, and by repeated sampling in the same session. Minimal complications were detected in our study being pneumothorax and spread of the tumor cells (2.35% for each) that signifies its safety. Honestly, our work lacks some standpoints, first; studying small number of cases but owed to cautious selection of cases as well as the need to study different types of pleural pathological lesion other than those findings. Second the need to use high frequency transducers in order to assess the more detailed echographic criteria for each lesion.
Conclusion
Yet, transthoracic ultrasound symbolizes the third clinician eye in favoring the probable clinical diagnosis of exudative pleural effusion not skipping over the pathology role in establishing the final diagnosis. Ultrasound-guided pleural forceps biopsy being a simple, efficient, and safe procedure makes its way among minimal invasive thoracic procedure with high success rate. It cannot be carried out effortlessly and securely in very sick and obese disabled and skeletally deformed patients.
References
- Beckh S, Bolcskei PL, Lessnau KD. Real-time chest ultrasonography. A comprehensive review for the pulmonologist. Chest. 2002; 122: 1759-1773.
- Reuss J. Sonographic imaging of the pleura: nearly 30 years experience. Europ J Ultrasound. 1996; 3: 125-139.
- Ziegler CM, Seitz K, Leicht-Biener U, Mauch M. Detection of therapeutically relevant diagnoses made by sonography of the upper abdomen: portable versus high-end-sonographic units — a prospective study. Ultraschall Med. 2004; 25: 428-432.
- Yang PC, Sheu JC, Luh KT, Kuo SH, Yang SP. Clinical application of real time ultrasonography in pleural and subpleural lesions. J Formosan Med Assoc. 1984; 83: 646-657.
- Saito T, Kobayashi H, Kitamura S. Ultrasonographic approach to diagnosing chest wall tumors. Chest 1988; 94: 1271-1 273.
- Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR. 1981; 136: 297-301.
- Mc Loud TC, Flower CDR. Imaging the pleura: sonography, CT, and MR imaging. AiR. 1991; 156: 1145-1153.
- Henschke CL. The pathogenesis, radiologic evaluation and therapy of pleural effusion. Radiol Clin North Am. 1989; 27: 1241 -1255.
- Menzies R, Charbonneau M. Thoracoscopy for the diagnosis of pleural disease. Ann Intern Med. 1991; 114: 271-276.
- Ryan C, Rodgers R, Unni K. The outcome of patients with pleural effusion of indeterminate cause at thoracotomy. Mayo Clin Proc. 1981; 56: 145-149.
- Yu CJ, Yang PC, Chang DB. Diagnostic and therapeutic use of chest sonography: value in critically ill patients. Am J Roentgenol. 1992; 159: 695-701.
- Yang PC, Luh KT, Sheu JC. Peripheral pulmonary lesions: biopsy. Radiology. 2008; 455: 450-456.
- Seitz K, Pfeffer A, Litlmann M. Ultrasound guided forceps biopsy of the pleura. Ultraschall Med. 1999; 20: 60-65.
- Blank W. Interventional chest sonopraphy. Mathis G, editor. In: Chest Sonography. 2nd Edn. Springer-Verlag. Berlin, Heidelberg. 2008; 184-205.
- Light RW. Pleural diseases. 4th Edn. Philadelphia: Lippincott Williams and Wilkins. 2001; 40-74.
- Mathis G, Blank W, Reisig A. Physical principals of ultrasound. Mathis G, Reus J, editors. In: Chest sonography. 1st Edn. Berlin, Springer. 2008; 45-56.
- Maskell NA, Davies CWH, Nunn AJ. U.K. controlled trial of intrapleural streptokinase for pleural infection (MIST1). NEJM. 2005; 352: 865-874.
- Adams RF, Gray W, Davies RJ, Gleeson FV. Percutaneous image guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest. 2001; 120: 1798-1802.
- Heilo A, Stenwig AE, Solheim P. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology. 1999; 211: 657-659.
- Bittner RC, Schnoy N, Schonfeld N. Hochauflosende Magnetresonanztomographie (HR-MRT) von Pleura und Thoraxwand: Normalbefund und pathologische Veonderungen. Fortschr Rontgenstr. 1995; 162: 296-303.
- Gorg C, Bert T, Gorg K. Contrast-enhanced sonography for differential diagnosis of pleurisy and focal pleural lesions of unknown cause. Chest. 2005; 128: 3894-3899.
- Reu BJ, Schneider H. Thoraxw and prozesse und Lung enverschattungen. Rettenmaier G, Seitz K, editors. In: Sonographische Differential diagnostik. Weinheim: VCH Verlagsgesellschaft. 1992; 803- 832.
- Gorg C, Schwerk WB, Gorg K, Walters E. Pleural effusion: An "acoustic window" for sonography of pleural metastases. J Clin Ultrasound. 1991; 19: 93-97.
- Yang PC, Chang DB, Lee YC, Yu CJ, Kuo SH, Luh KT. Mediastinal malignancy: Ultrasound guided biopsy through the supraclavicular approach. Thorax. 1992b; 47: 377-380.
- Herth F, Schmitteckert H, Schulz M, Becker HD. Diagnostik des Pneumothorax mittels transthorakalem Ultraschall—eine prospektive Untersuchung. Ultraschall in Med. 2003; 24: S30.
- Gehmacher O, Kopf A, Scheier M, Bitschnau R, Wertgen T, Mathis G. Ist eine Pleuritis sonographisch darstellbar? Ultraschall in Med. 1997; 18: 214-219.
- Loddenkemper R. Pleuraerkrankungen. Ferlinz R, editor. In: Pneumologie in Praxis und Klinik. Thieme, Stuttgart, New York. 1994; 712-717.
- Mowé G, Tellnes G. Malignant pleural mesothelioma in Norway 1960-1992. Tidsskr Nor Laegeforen. 1995; 115: 706-709.
- Wernecke K. Ultrasound study of the pleura. Eur Radiol. 2001; 10: 1515-1523.
- Sugarbaker DJ, Jaklitsch MT, Liptay MJ. Mesothelioma and radical multimodality therapy: who benefits? Chest. 1995; 107: 345-350.
- Canto A, Rivas J, Saumench J. Points to consider when choosing a biopsy method in cases of pleurisy of unknown origin. Chest. 1983; 84: 176-179.
- Poe R, Israel R, Utell M. Sensitivity, specificity and predictive values of closed pleural biopsy. Arch Intern Med. 1984; 144; 325-328.
- Prakash V, Reiman H. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc. 1985; 60: 158-164.
- Gowie R, Escreet B, Goldstien B, Langton ME, Leigh RA. Pleural biopsy: a report of 750 biopsies performed using Abrams pleural biopsy punch. S Afr J. 1983; 64; 92-95.
- Sahn S. The pleura. Am Rev Respir Dis. 1988; 138: 184-234.
- Crossno J. Procedures in pulmonary medicine. In: Current Diagnosis and Treatment in Pulmonary Medicine. Mc Graw- Hill Companies Inc. 2003; 57-66.
- Mc lean A, Bicknell S, Mc Alpine L, Peacock AJ. Investigation of pleural effusion; an evaluation of the new Olympus LTF semiflexible thoracofiberscope and comparison with Abram’s needle biopsy. Chest. 1998; 114 : 150-153.
- Agmy G, Ahmed Y, Shaaban LH, Kamal N. Ultrasound-guided forceps for pleural biopsy. Egyptian Journal of Chest Diseases and Tuberculosis. 2014; 63: 363-368.