
Review Article
Austin J Pulm Respir Med. 2022; 9(1): 1085.
Bronchial Asthma: Etiology, Pathophysiology, Diagnosis and Management
Bereda G*
Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia
*Corresponding author: Gudisa Bereda, Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia; Email: gudisabareda95@gmail.com; Tel: +251913118492/+1919622717
Received: December 13, 2021; Accepted: January 21, 2022; Published: January 28, 2022
Abstract
Bronchial asthma is a disorder of the airways causing swelling and narrowing; which leads to wheezing, shortness of breath, chest tightness, and coughing. The impact of asthma in children depends on complex interaction between disease severity, reaction of children towards disease, treatment efficiency, social roles, and social environment. Most common asthma triggers of bronchial asthma are dust, animal dander, weather changes, pollution, mold, pollen, respiratory infections, stress, and tobacco smoke. The main pathophysiological characteristics of asthma are inflammation and airway remodeling, which include goblet cell hyperplasia, subepithelial fibrosis, collagen deposition, mucosal gland, hyperplasia, smooth muscle hypertrophy, and changes in the extracellular matrix. Spirometry (test lung function when diagnosing asthma), pulse oximetry (monitors oxygen saturation which used to measure amount of arterial hemoglobin that is combined with oxygen) used for diagnosis bronchial asthma. The goal of asthma treatment is to achieve normal respiratory function, with an absence of symptoms, exacerbations, or adverse effects. The beta 2 agonists are sympathomimetic drugs that produce “selective” activation of beta 2 adrenergic receptors, promote bronchodilation, and thereby relieve bronchospasm. The use of short actin beta 2 agonists as a reliever five or more times daily indicates controller agents need to be increased. Prednisone and prednisolone are preferred glucocorticoids for oral therapy of asthma. Methylxanthines are widely used in the treatment of asthma due its ability to inhibit phosphodiesterase causing bronchodilatation. The adverse effects of theophylline include gastrointestinal symptoms such as nausea and vomiting at initial oral administration. In addition, toxic symptoms may progress to tachycardia and arrhythmia.
Keywords: Bronchial asthma; Diagnosis; Etiology; Pathophysiology; Management
Abbreviations
BA: Bronchial Asthma; CysLT1: Cysteinyl Leukotriene 1; FEV1: Forced Expiratory Volume; GINA: Global Initiative for Asthma; GWA: Genome-Wide Association; HPA: Hypothalamic Pituitary Adrenocortical; ICS: Inhaled Corticosteroids; LABA: Long-Acting B2 Agonist; LAMA: Long-Acting Muscarinic Antagonist; LTRA: Leukotriene Receptor Antagonists; PDEI-5: Phosphodiesterase Inhibitors 5; PEF: Peak Expiratory Flow; QOL: Quality of Life; TGF: Transforming Growth Factor; TH-2: T-Helper Cell 2; SABA: Short- Acting B2 Agonist; SCIT: Specific Subcutaneous Immunotherapy; SNPs: Single Nucleotide Polymorphisms;
Introduction
Bronchial asthma is a chronic inflammatory disease of the respiratory passages, occurring with the participation of mast cells, eosinophils and T-lymphocytes, the release of a large number of inflammatory mediators. Inflammation of the respiratory passages causes their hyperreactivity, bronchial obstruction, and respiratory symptoms [1,2]. Airway obstruction in bronchial asthma is mainly caused by the following four mechanisms: i) contraction of bronchial smooth muscle; ii) edema of the airway walls; iii) mucous plugging of the bronchioles; iv) irreversible changes in the lungs (“remodeling”) [3]. Bronchial asthma is a major public health problem affecting a large number of individuals of all ages. Globally, 100-150 million people suffer from asthma [4]. In general, the prevalence of asthma is higher in developed countries than in developing countries, which is a serious public health problem in all ages [5]. Incidence of asthma in adults is 3.8%/1000 at-risk adults. Incidence of asthma in children is 12.5%/1000 at-risk children, especially among children 0-4 years old, the incidence is 23.4%/1000 children. The World Health Organization recognizes asthma as a major health problem. Asthma can occur at any age but children and young adults are the commonly affected age groups. Both sexes are affected almost equally though slight differences in prevalence between males and females have been reported. Although asthma cannot be ‘‘cured,’’ clinical episodes can largely be prevented and controlled by proper management [6]. The impact of asthma in children depends on complex interaction between disease severity, reaction of children towards disease, treatment efficiency, social roles, and social environment. Bronchial asthma, if a remains uncontrolled during childhood leads to continuous symptoms leading to limitations in physical activities and it can lead to development of chronic obstructive pulmonary disease during the later years of life [7-9]. Long-standing inflammation will damage airways, and induces airway remodelling, entailing subepithelial fibrosis under the basement membrane, smooth muscle hypertrophy, and submucosal gland hyperplasia. This results in intractable asthma, presenting irreversible airflow limitation and persistent airway hyperresponsiveness [10]. The airways of asthmatic individuals are characterized by a T-Helper cell (Th)-2-profile inflammation consisting of an overabundance of eosinophils, mast cells and Th2 lymphocytes. These inflammatory cells release mediators that trigger bronchoconstriction, mucous secretion and, possibly, remodeling. The number of infiltrating leukocytes, such as mast cells, eosinophils, CD8+ and CD45+ T cells, correlates with AHR in patients treated with Inhaled Corticosteroids (ICS). The inflammatory mediators that drive this process include the Th2 cytokines Interleukin (IL)- 4, IL-5, IL-9 and IL-13, Transforming Growth Factor (TGF)-beta, Granulocyte/Macrophage Colony Stimulating Factor (GM-CSF), lipid mediators and histamine. Some of these mediators, such as TGFbeta, IL-11 and IL-17, have potent remodelling properties. Histamine was recently proposed to participate in airway remodelling through increased fibroblast proliferation and connective tissue growth factor production [11-13] (Figure 1).
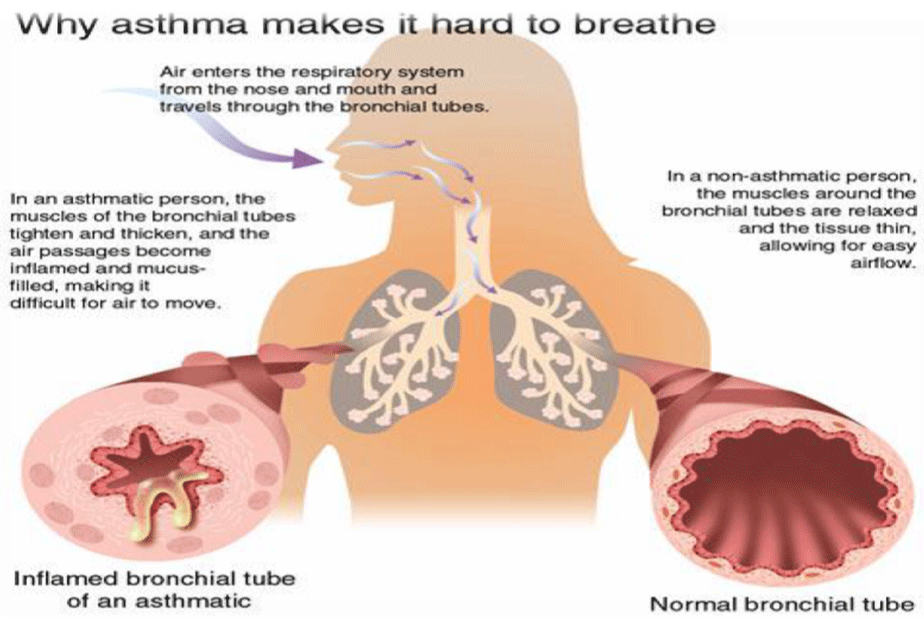
Figure 1: How bronchial asthma cause breathe difficulties.
Etiology of Bronchial Asthma
The etiology of asthma is multifactorial, and its clinical picture varies greatly among patients. Some asthmatic patients demonstrate airway inflammation, predominantly involving neutrophils.
Although the etiology of bronchial asthma remains unknown, its pathogenesis is well believed to be affected by both genetic and environmental factors. Environmental substances that may cause asthma include indoor allergens (e.g., dust mites, pets, and cockroaches), outdoor allergens (e.g., pollen and dust), and sources of infection (e.g., bacteria, fungi, and parasites), occupational pollutants, or food additives. In terms of genetic factors, a Genome- Wide Association (GWA) study identified more than 100 genes as being significantly associated with the onset of bronchial asthma. In addition, mutations in the ORM1 gene have been revealed in studies on Single Nucleotide Polymorphisms (SNPs) to also be closely related to the onset of asthma [14-16].
Day-night pattern in bronchial asthma: Bronchial asthma is worse at night than during the day in most asthmatic persons. This disease is a burden, and men suffer more than women and elderly more than the young during winter and at night more than during the day or in spring [17].
Mechanisms of nocturnal bronchial asthma: The mechanisms of nocturnal bronchial asthma are complex and involve the daytime antigen-provoked release of pro-inflammatory mediators from mast and eosinophil cells over the span of several hours thereafter, which results by the end of the day in the exacerbation of inflammation, smooth muscle bronchospasm and contraction, and over-stimulation of mucus glands with mucus hypersecretion of the small airways of the lung. All these processes can be modulated by neuroendocrine and other important high amplitude circadian rhythms, such as those of the Hypothalamic Pituitary Adrenocortical (HPA) and autonomic nervous systems [17].
Pathophysiology of Bronchial Asthma
The main pathophysiological characteristics of asthma are inflammation and airway remodeling, which include goblet cell hyperplasia, subepithelial fibrosis, collagen deposition, mucosal gland, hyperplasia, smooth muscle hypertrophy, and changes in the extracellular matrix. These changes can result in immune system imbalance, eventually leading to airway hyperresponsiveness. Throughout the course of bronchial asthma, changes in the levels of transcription factors, inflammatory mediators, chemokines, cytokines, and cell apoptosis and proliferation related proteins also take place [14,18,19] (Figure 2).
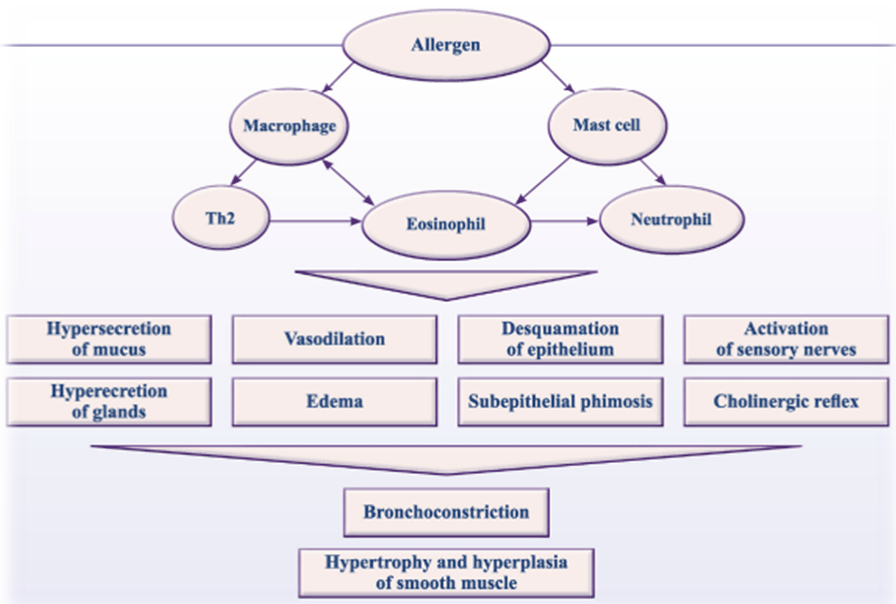
Figure 2: Inflammation plays the central role in bronchial asthma pathogenesis.
Clinical Manifestations of Bronchial Asthma
The symptoms of bronchial asthma include dyspnea (difficulty in breathing), rapid respiration, wheezy chest, chest tightness, acute bronchoconstriction (immediate), and croupy cough. Bronchial asthma is seldom a problem for those who suffer from the mildest form of the disease. However, it is a significant medical condition, and even life threatening, for those who are prone to severe bronchial asthma. Bronchial asthma is characterized by persistent airway inflammation, heightened airway hyperreactivity to antigens and other environmental agents, and markedly reduced and abnormal airway caliber. Symptoms can happen each time the airways are irritated [17].
Diagnosis of Bronchial Asthma
The criteria for diagnosis of asthma were: i) reversible airway obstruction that varied markedly, both spontaneously and with treatment; ii) intermittent experience of wheeze (usually worse on expiration and characteristically relieved by inhaled beta 2-agonists), cough (usually unproductive), shortness of breath (not always associated with wheeze) and chest tightness; iii) the possibility of being triggered by a number of factors, including allergens, irritants, physical factors, emotions, occupational agents, food additives, changes in the weather, endocrine factors and upper respiratory tract viral infections; and iv) a reduction in Forced Expiratory Volume in one second (FEV1; absolute value and/ or percentage of predicted value) and/or Peak Expiratory Flow (PEF) during the attacks [20]. Spirometry (test lung function when diagnosing asthma), Pulse Oximetry (monitors oxygen saturation (SpO2) which used to measure amount of arterial hemoglobin that is combined with oxygen) used for diagnosis bronchial asthma.
Differential Diagnosis of Bronchial Asthma
The following entities should be considered in the differential diagnosis of bronchial asthma because of their frequency and clinical significance: chronic obstructive pulmonary disease; hyperventilation; aspiration; laryngeal changes/vocal cord dysfunction; pneumothorax; cystic fibrosis; cardiac diseases, e.g., left heart failure; pulmonary embolism; gastroesophageal reflux disorder. In as many as 10% to 20% of cases, a clear-cut distinction between asthma and chronic obstructive pulmonary disease cannot be drawn [10].
Treatment of Bronchial Asthma
The goal of asthma treatment is to achieve normal respiratory function, with an absence of symptoms, exacerbations, or adverse effects. Because normal respiratory function cannot be restored in patients with airway remodeling, it can be assessed based on their best values [10]. The most effective treatment for asthma is identifying triggers, such as smoke, pets, or aspirin and eliminating exposure to them. Medical treatments used depend on the severity of illness and the frequency of symptoms [21]. Approach to asthma treatment is used for prevention of the exposure to antigen; reduction of bronchial inflammation and hyperactivity; dilatation of the narrowed bronchi of the lung. Consummate antiasthma drugs can be administered by inhalation, a route with three obvious advantages: i) enhance therapeutic effects (by delivering drugs directly to their site of action), ii) systemic effects are minimized, and iii) relief of acute attacks is rapid.
Non-Pharmacological Treatment of Bronchial Asthma
The removal of allergens, especially of pets with feathers or fur, is an important element of non-pharmacological treatment. Lifestyle modification should include regular balanced diet, stop smoking, avoid sedentary life style because sedentary lifestyle cause decreases lung strength and avoidance of obesity or weight reduction because obesity cause increases overall inflammatory response of the human body. The restriction of physical exercise is not advisable rather asthmatics should be encouraged to participate in exercises. Improved self-management leading to better symptomatic control, reduction of the number of asthma attacks and emergency situations, and improvement in various other parameters of disease course including days taken off from school or work and days spent in hospital; physical training (reduction of asthma symptoms, improved exercise tolerance, improved quality of life, reduced morbidity); respiratory therapy and physiotherapy (e.g., breathing techniques, pursed-lip breathing); smoking cessation (with medical and non-medical aids, if necessary); psychosocial treatment approaches (family therapy).
Pharmacological Treatment of Bronchial Asthma
Asthma agents consist of two types of drugs; long-term controller agents are used continuously for long-term management (controllers) and reliever agents are used in the short term to treat asthma symptoms (relievers). Controllers are defined as “regular-use agents aimed at achieving good control” and relievers are defined as “rescue-use agents aimed at treating asthma exacerbations”. They are administered orally, via inhalation, injection (drip infusion, subcutaneous, or intramuscular), or skin patches. Inhaled agents can directly access the inflammatory sites, with increased local concentration; therefore, the agents are remarkably effective and allow the systemic concentration of the drugs to be maintained at lower levels, so that systemic side effects can be lower frequencies [10]. Global Initiative for Asthma (GINA), however, the classification system is based on the degree of clinical control that has been achieved, ranging from “controlled” to “partly controlled” to “uncontrolled”. This new classification is meant to emphasize the point that the severity of asthma depends not just on the severity of the underlying illness itself, but also on its response to treatment. Furthermore, the severity of asthma can fluctuate considerably over a period of months to years. GINA’s Global Strategy defines clinically controlled asthma as follows: no daytime symptoms at all, or at most two times per week; no limitation of the activities of everyday living, including physical exercise; no symptoms at night, or no awakening because of asthma; no need for rapidly-acting bronchodilators for symptomatic treatment (“relievers”), or at most two times per week; normal or nearly normal pulmonary function; no exacerbations [10] (Table 1).
Treatment step 1
Treatment step 2
Treatment step 3
Treatment step 4
Long-term management agents
Basic treatment
Inhaled corticosteroid (low dose)
Inhaled corticosteroid (low to medium doses)
Inhaled corticosteroid (medium to high doses)
Inhaled corticosteroid (high dose)
If the above agent cannot be used, use one of the following agents.
LTRA Theophylline sustained release preparation (unnecessary for rare symptoms)If the above agent is ineffective, concomitantly use one of the following agents.
LABA (a compounding agent can be used)
LTRA Theophylline sustained release preparationConcomitantly use one or more of the agents below. LABA (a compounding agent can be used)
LTRA Theophylline sustained release preparation LAMAConcomitantly use multiple agents of those below.
LABA (a compounding agent can be used)
LTRA Theophylline sustained release preparation LAMA
Anti-IgE antibody
Oral corticosteroidAdditional treatment
Anti-allergics other than LTRA
Anti-allergics other than LTRA
Anti-allergics other than LTRA
Anti-allergics other than LTRA
Exacerbation treatment
Inhaled SABA
Inhaled SABA
Inhaled SABA
Inhaled SABA
Table 1: Treatment steps for bronchial asthma [3].
• Antiallergics refer to mediator antireleasers, histamine H1 antagonists, thromboxane A2 inhibitors, and Th2 cytokine inhibitors.
• Anti-IgE antibody is indicated for patients who are positive for perennial inhaled allergen with serum total IgE value within 30e1500 IU/ml.
• Oral corticosteroids are intermittently administered for a short period. Maintain the minimum maintenance dose if a patient cannot be controlled by enhanced treatment with other agents and short intermittent administration.
• Management against mild exacerbations is shown. For other exacerbations.
• In patients treated with a combination of budesonide/ formoterol as a controller, if used as a rescue, the agent should not be used beyond the maximum number of uses per time and per day. The maximum number of uses is generally up to 8 inhalations/day; however, temporarily, it can be used up to 12 inhalations/day (for 3 days: budesonide, 1920 mg/day; formoterol 54 mg/day). When more than 8 inhalations/day of budesonide/formoterol are needed, a physician should be consulted.
• Soft mist inhaler of tiotropium.
• Anti-IgE antibody and oral corticosteroid are considered when asthma control cannot be achieved with inhaled corticosteroid plus LABA and LTRA, etc.
Agents for Short-Term Management (Reliever)
Short acting beta 2 agonists (SABAs)
SABAs are regarded as reliever agents. Inhalation therapy using a pMDI, DPI, and nebulizer shows a comparable or even higher bronchodilator action than with oral administration. Examples of inhalation short acting beta 2 agonists are albuterol, bitolterol, pirbuterol, terbutaline, salbutamol, levalbuterol, mesylate. The beta 2 agonists are sympathomimetic drugs that produce “selective” activation of beta 2 adrenergic receptors, promote bronchodilation, and thereby relieve bronchospasm. In addition, they suppress histamine release in the lung and increase ciliary motility. Beta2- selective agents have replaced older, less selective sympathomimetics (eg, epinephrine, isoproterenol) for asthma therapy. All patients with asthma use these drugs. Beta2 agonists, given by inhalation, are the most effective drugs available for relieving acute bronchospasm and preventing Exercise-Induced Bronchospasm (EIB). The increasing need for the use of a SABA can be regarded as loss of asthma control. Short-acting inhaled preparations, effects begin almost immediately, peak in 30 to 60 minutes, and persist for 3 to 5 hours. Because of this time course, the Short-Acting Beta 2 Agonists (SABAs) can be used to abort an ongoing attack, but cannot be used for prolonged prophylaxis. The use of a SABA as a reliever five or more times daily indicates controller agents need to be increased. When an asthma attack occurs, 1 or 2 puffs of SABAs are administered. If the effects are not satisfactory after repeated inhalation every 20 min for 1 hr, medical consultation is needed. Short acting inhaled preparations taken PRN (as needed) to relieve an ongoing attack and with EIB, they are taken before exercise to prevent an attack. For patients undergoing a severe acute attack, a nebulized SABA is the treatment of choice [10]. Short acting beta-2 agonists should be used prior to anticipate exercise in a patient with exercise-induced asthma to alleviate symptom. When used acutely (<10 days), even in very high doses, do not cause significant adverse effects. However, prolonged therapy, even in moderate doses, can be hazardous. Potential adverse effects include adrenal suppression, osteoporosis, hyperglycemia, peptic ulcer disease, and, in young patients, suppression of growth. There are a few adverse effects, such as stimulation of the cardiovascular system, skeletal muscle tremor, and hypokalemia.
Oral corticosteroids
An oral corticosteroid together with a SABA needs to be administered for about 1 week in moderate exacerbations. Prior short-term treatment of asthma symptoms (usually less than 1 week) with a dose of an oral corticosteroid (approximately 0.5 mg/kg of prednisolone) prevents acute exacerbations, reduces emergency visits and hospital admissions, and improves daily life. Prednisone and prednisolone are preferred glucocorticoids for oral therapy of asthma. For acute therapy, the usual adult dosage for either drug is 30 to 40 mg twice daily for 5 to 7 days. For long-term treatment, alternateday dosing is recommended (to minimize adrenal suppression). Oral glucocorticoids are reserved for patients with severe asthma. Because of their potential for toxicity, these drugs are prescribed only when symptoms cannot be controlled with safer medications (inhaled glucocorticoids, inhaled beta 2 agonists). Because the risk of toxicity increases with duration of use, treatment should be as brief as possible. Adherence to the asthma drugs and inhalation technique should be checked and changes of controllers and addition of other agents should be considered when the asthmatic patient has frequent exacerbations requiring short-term oral corticosteroids. In general, viral infection is more often involved in asthma exacerbations than bacterial infection, and short-term treatment is not likely to cause serious infection. In short-term treatment, a sudden dose reduction or discontinuation of treatment will not result in adrenocortical insufficiency (i.e., steroid withdrawal syndrome). When oral glucocorticoids used acutely (<10 days), even in very high doses, do not cause significant adverse effects. However, prolonged therapy, even in moderate doses, can be hazardous. Potential adverse effects include adrenal suppression, osteoporosis, hyperglycemia, peptic ulcer disease, and, in young patients, suppression of growth.
Theophylline
Aminophylline is usually used as a drip in vessels or continuous drip in vessels in case of a reliever. Because of its narrow therapeutic range, aminophylline should be used by monitoring the serum levels. Aminophylline produces bronchodilation by relaxing smooth muscle of the bronchi, probably by blockade of receptors for adenosine. Theophylline is the principal methyl-xanthine employed in asthma. Benefits derive primarily from bronchodilation. Aminophylline is a theophylline salt that is considerably more soluble than theophylline itself. The pharmacologic properties of aminophylline and theophylline are identical. Intravenous administration is employed most often. Infusions should be done slowly (no faster than 25 mg/min), because rapid injection can produce severe hypotension and death. In Europe and the United States, administration of aminophylline is not recommended for exacerbations, considering its effectiveness and side effects.
Short-acting muscarinic receptor antagonists (SAMAs)
SAMAs have additive effects with beta 2 agonists and reduce the rate of hospital admissions and improve pulmonary function for moderate to severe exacerbations. Although the onset of bronchodilatory effects of SAMAs is slower than that of SABAs, SABAs are available for acute exacerbations when SABAs are unavailable [10,22,23].
Agents for Long-Term Management (Controllers)
Controllers are considered as agents for alleviating and eliminating asthma symptoms, and normalizing and maintaining respiratory functions. They have anti-inflammatory effects and/or long-term bronchodilatory effects, and are classified based on their mechanisms of action.
Corticosteroids (Steroids)
Corticosteroids are currently the most important and effective anti-inflammatory agents among asthma treatments. The main mechanisms of action include (i) inhibition of inflammatory cell infiltration into the lungs, airways and inhibition of migration and activation of inflammatory cells; (ii) reduction of vascular permeability; (iii) suppression of airway secretion; (iv) inhibition of airway hyper-responsiveness; (v) inhibition of cytokine production; (vi) promotion of the effects of b2-agonists; (vii) inhibition of arachidonic acid metabolism in cells other than human mast cells and the production of leukotrienes and prostaglandins. Currently, four forms of steroids are available: intravenous, intramuscular, oral, and inhaled. Steroids used for long-term management of asthma are usually ICSs, because these have a lower risk of side effects. When control of asthma cannot be achieved with ICSs and ICSs plus other asthma drugs, such as bronchodilators, or in patients with complications, an oral corticosteroid only should be used. An aqueous suspension of triamcinolone acetonide in an intramuscular injection should not be used, because of its adverse effects. ICSs have been reported to (i) improve asthma symptoms; (ii) improve QOL and respiratory function; (iii) alleviate airway hyperresponsiveness; (iv) suppress airway inflammation; (v) reduce the frequency and severity of exacerbations; (vi) reduce the maintenance dose of ICSs for a long period of time; (vii) reduce the medical expenses associated with asthma; (viii) suppress airway remodeling; and (ix) reduce the mortality rate due to asthma. Once asthma symptoms have developed, early administration of an ICS (early intervention) will decrease the frequency of acute exacerbations. However, asthma cannot be cured by ICS treatment. If the treatment is discontinued, asthmatic symptoms cannot be controlled [10,24]. Adverse effects inhaled glucocorticoids: These preparations are largely devoid of serious toxicity, even when used in high doses. The most common adverse effects are oro-pharyngeal candidiasis and dysphonia (hoarseness, speaking difficulty). Both effects result from local deposition of inhaled glucocorticoids. To minimize these effects, patients should; i) gargle after each administration and ii) employ a spacer device during administration, which will greatly reduce drug deposition in the oropharynx. If candidiasis develops, it can be treated with an antifungal drug. With long-term, high-dose therapy, some adrenal suppression may develop, although the degree of suppression is generally low. In contrast, with prolonged use of oral glucocorticoids, adrenal suppression can be profound. Can also promote bone loss at least in premenopausal women, but much lower than oral glucocorticoids. To minimize bone loss, patients should; i) Use the lowest dose possible, ii) Ensure adequate intake of calcium and vitamin D, and iii) participate in weight-bearing exercise. Glucocorticoids can slow growth in children and adolescents but do not decrease adult height.
Long-acting b2 agonists (LABAs)
β2-adrenergic agonist medication β2-agonist medications are often prescribed to manage bronchial asthma. They bind to cell membranes and activate adenylate cyclase, resulting in relaxation of the smooth muscle of the small airways so as to increase airway caliber and airflow rate to make breathing easier. β2-agonists may also modulate airway inflammation. Long-acting inhaled beta 2 agonists used for patients who experience frequent attacks can inhale a LABA for long-term control and dosing is done on a fixed schedule, not PRN. LABAs are not first-choice agents for long-term control, and should not be used alone. Rather, they should be added to the regimen when control has been inadequate with an inhaled glucocorticoid. Early generation conventional aerosol bronchodilator medications have a limited-effect duration, generally no more than 4 hr; thus, they are unsuitable for NBA; new-generation bronchodilator agents, in comparison, are effective for 12 to 24 hr. Salmeterol Xinafoate (SM) is an inhaled LABA that cannot be used as monotherapy for the treatment of asthma; however, it has strong synergistic effects when combined with an ICS. Conventional, short-acting β2-agonist aerosol medications are relied upon to alleviate acute asthma crises; however, as a monotherapy they afford limited or no protection against NBA [17,25]. Adverse effects inhaled beta2 agonists: Inhaled beta2 agonists are well tolerated. Systemic effects (tachycardia, angina, & tremor) can occur, but are usually minimal. If dosage of oral preparations is excessive, stimulation of cardiac beta1 receptors can cause angina pectoris and tachydysrhythmias. Patients should be instructed to report chest pain or changes in heart rate or rhythm and tremor (by activating beta2 receptors in skeletal muscle). Tremor can be reduced by lowering the dosage or spontaneously.
Sustained-release theophylline
Methylxanthines are widely used in the treatment of asthma due its ability to inhibit Phosphodiesterase (PDE) causing bronchodilatation. Methylxanthines also have anti-inflammatory, immunomodulatory and bronchoprotective effects in addition to bronchodilation. These drugs require therapeutic drug monitoring because of narrow margin of safety requiring strict monitoring of its blood levels. Methylxanthines were found to have no added significant effect over inhaled beta-agonists. Has a narrow therapeutic range, and hence dosage must be carefully controlled. With regular use, theophylline can decrease the frequency and severity of asthma attacks. Because its effects are prolonged, theophylline may be most appropriate for patients who experience nocturnal attacks. Intravenous theophylline has been employed in emergencies. In the past, theophylline was a first-line drug for asthma and nearly all patients with chronic asthma took it. However, use of theophylline has declined sharply, largely because we now have safer and more effective medications. According to latest guidelines Methylxanthines have restricted role in the management of asthma exacerbations in view of their poor safety profile in comparison to short acting beta agonistic agents. Theophylline, a PDE inhibitor has been used in asthma for its antiinflammatory effect in the concentration range of 5- 20 μg/ml but with a variety of side effects above >20 μg/ml [21,26,27]. Phosphodiesterase 5 inhibitors may be a therapeutic option in asthma treatment because they increase intracellular concentrations of cyclic adenosine monophosphate, which has both bronchodilatory and anti-inflammatory effects on inflammatory cells involved in the pathogenesis of asthma. A new thalidomide analogue (a potent inhibitor of two main PDE isotypes in the lungs PDE4 and PDE5) has been shown to be as effective as dexamethasone in inhibiting inflammatory changes in airways, and preventing parenchyma and airway remodelling in a murine model of chronic asthma. A PDE3 inhibitor (siguazodan) has been shown to reduce in vitro proliferation of human airway smooth muscle cells, while the PDE4 inhibitor (roflumilast) reduced inflammation, subepithelial collagen deposition and thickening of airway epithelium in a murine asthma model [11,28,29]. The adverse effects of theophylline include gastrointestinal symptoms such as nausea and vomiting at initial oral administration. In addition, toxic symptoms may progress to tachycardia and arrhythmia. In the most severe cases, convulsions may occur that can lead to death.
Leukotriene receptor antagonists (LTRAs)
Montelukast, a Cysteinyl Leukotriene 1 (CysLT1) receptor antagonist is commonly used in asthma therapy as an add-on treatment. It has also been recently approved for the treatment of allergic rhinitis and exercise induced asthma. In a clinical study, asthmatic patients with nasal polyposis treated with montelukast experienced a 70% improvement in nasal symptoms and a 60% to 90% improvement in clinical asthma scores. Montelukast decreases sputum eosinophils after allergen challenge in asthmatic subjects. In addition to their anti-inflammatory effects, CysLT antagonists may play an important role in the pathogenesis of airway remodelling. Montelukast has been shown to significantly inhibit ovalbumininduced airway smooth muscle hyperplasia, mucus gland hyperplasia and subepithelial fibrosis in sensitized mice. LTRAs have a bronchodilator action and inhibit airway inflammation, resulting in significant improvement of asthma symptoms and respiratory function; allow as-needed inhalation of a beta 2 agonist, reduce airway inflammation, airway hyperresponsiveness, the dosage of ICSs, and asthma exacerbations; and improve patients’ QOL. LTRAs are useful for long-term management of patients with asthma complicated by allergic rhinitis, Exercise-Induced Asthma (EIA), and Aspirin-Exacerbated Respiratory Disease (AERD). Generally, LTRA monotherapy is less effective than that with low-dose ICSs in mild asthmatic patients. A recent study reported a decreased lymphocyte and myofibroblast count in the airways of asthmatic subjects after only eight weeks of montelukast treatment. Evidence from these animal and human studies indicates that antileukotrienes may prevent airway remodelling at the level of goblet and smooth muscle cell hyperplasia, and subepithelial fibrosis. However, long-term studies are needed to confirm the clinical outcomes of the antiremodelling effect of CysLT1 receptor antagonists in asthmatic patients [11,30-32].
Combination agents comprised of ICS and inhaled LABA (ICS/LABA)
Inhaled corticosteroids and long-acting β-receptor agonists are the first-line therapy in the treatment of asthma. However, some people have poor compliance because of the adverse reactions of glucocorticoids, which leads to uncontrolled of asthma in this part of population and repeatedly seeks medical help, wasting large medical resources. Besides, some asthma patients have long-term regular medications and usually have few symptoms, but there are intermittent or mild asthma attacks [5,31,32]. As fixed combinations of ICS with LABA have become available over the last few years, new concepts for the treatment of asthma have been developed and clinically tested, with the goal of better control of bronchial asthma. These concepts take the varying pharmacological properties of the LABA into account (e.g., the rapid onset of activity of formoterol and the delayed onset of activity of Salmeterol. Furthermore, ICS/ LABA allows reduction of the ICS dose, and improves the control of asthma. Combination of an ICS and LABA is more effective than an ICS with sustained-release theophylline. The advantages of the ICS/ LABA combination are (i) the number of inhalations can be reduced; (ii) excellent adherence can be achieved; and (iii) the use of LABAs alone can be avoided [3].
Long-acting anti-muscarinic receptor antagonist (LAMA)
Anticholinergic agent’s cholinergic tone increases during the night and may contribute to the worsening of NBA. Moreover, the amplitude of the circadian rhythm in vagal tone might be amplified in NBA due to exacerbation of airway inflammation and associated up-regulation of muscarinic receptors overnight. Reports on the therapeutic effect of anticholinergic medications on NBA are inconsistent; some investigations found them to moderate NBA and to attenuate the nocturnal decline of airway caliber, while others have not. Since vagal tone differs greatly during the 24 h, being higher during the sleep than activity span, perhaps the failure of traditional equal-interval, equal-dose regimens to impact airway caliber and protect against asthma overnight could be due to less than adequate dosing of this class of medications late in the day and/or at bedtime [17].
Anti-allergics other than LTRAs
Allergen-Specific Subcutaneous Immunotherapy (SCIT), also called “desensitization,” has been shown to reduce medication use and bronchial hyperreactivity, as compared with placebo, in mild to moderately severe asthma, although it does not improve pulmonary function values (evidence level A). This statement applies mainly to younger patients. SCIT has a markedly lower chance of success in older patients who have had asthma for a long time, whose symptoms arise independently of allergen exposure, and for whom anti-inflammatory pharmacotherapy has been less effective. SCIT is contraindicated in patients whose pulmonary function is persistently impaired with FEV1 values below 70%. Specific immunotherapy should be performed only by a physician with experience in allergology. It does not replace effective anti-asthmatic pharmacotherapy, but should rather be seen as a complementary element of asthma management. There is accumulating evidence that SCIT can help prevent the progression of allergic rhino-conjunctivitis to allergic asthma in children and adolescents [3,33]
Anti-IgE antibody: Anti-IgE antibody
Omalizumab is a humanized antihuman IgE monoclonal antibody. In Japan, Omalizumab is available for the following asthmatic patients: (i) those who have unstable asthmatic symptoms, even when treated with high doses of ICSs plus more than 1 controller agent; (ii) those who are positive for perennial inhaled antigens, such as house dust; (iii) the dose and frequency of administration are determined based on the dosage conversion table, according to the patient’s weight and serum IgE level (30e1500 IU/mL serum IgE). Omalizumab has the following effects in patients with poor asthma control, even despite treatment with a high dose of ICS: (i) it prevents exacerbation; (ii) reduces the frequency of asthmatic symptoms; (iii) improves QOL; (iv) reduces the frequency of emergency room visit and hospital admission; and (v) reduces the steroid dose. Omalizumab modestly improves FEV1 and PEF values. It has only been confirmed to be effective in poorly controlled patients treated with ICS/LABA. Omalizumab should be used as a therapeutic agent in step 4 treatment for severe persistent asthma. At 16 weeks after administration, the therapeutic effects can be comprehensively judged and it should then be determined whether the treatment needs to be continued [17, 34,35]
Mast cell stablizers
Cromolyn and nedocromil cromolyn and nedocromil are inhalational agents that suppress bronchial inflammation. Both drugs are used for prophylaxis-not quick relief in patients with mild to moderate asthma. Anti-inflammatory effects are less than with glucocorticoids. Cromolyn: Cromolyn is a very safe and effective drug for prophylaxis of asthma, but is not useful for aborting an ongoing attack. Administration is by inhalation and effects on the lung, cromolyn suppresses inflammation; it is not a bronchodilator. The drug acts in part by stabilizing the cytoplasmic membrane of mast cells, thereby preventing release of histamine and other mediators. In addition, cromolyn inhibits eosinophils, macrophages, and other inflammatory cells. Cromolyn is administered by inhalation. The fraction absorbed from the lungs is small (about 8%), produces no systemic effects, and excreted unchanged in the urine.
Conclusion
Bronchial asthma is a chronic inflammatory disease of the respiratory passages, occurring with the participation of mast cells, eosinophils and T-lymphocytes, the release of a large number of inflammatory mediators. Although the etiology of bronchial asthma remains unknown, its pathogenesis is well believed to be affected by both genetic and environmental factors. Approach to asthma treatment is used for prevention of the exposure to antigen; reduction of bronchial inflammation and hyperactivity; dilatation of the narrowed bronchi of the lung. Beta2 agonists, given by inhalation, are the most effective drugs available for relieving acute bronchospasm and preventing Exercise-Induced Bronchospasm (EIB). Glucocorticoids reduce asthma symptoms by suppressing inflammation. Specific anti-inflammatory effects of glucocorticoids include i) decreased synthesis and release of inflammatory mediators (eg, leukotrienes, histamine, prostaglandins); ii) decreased infiltration and activity of inflammatory cells (eg, eosinophils, leukocytes); iii) decreased edema of the airway mucosa (secondary to a decrease in vascular permeability). The advantages of the ICS/LABA combination are (i) the number of inhalations can be reduced; (ii) excellent adherence can be achieved; and (iii) the use of LABAs alone can be avoided.
Acknowledgments
The author acknowledged those who support him during preparation of this manuscript.
Data Sources: Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, and Cochrane database. Search terms included: definition, etiology, pathophysiology, diagnosis and management of bronchial asthma.
References
- Moskvin Sergey Vladimirovich and Khadartsev Aleksandr A. “Methods of effective low-level laser therapy in the treatment of patients with bronchial asthma,” BioMedicine. 2020; 10: 1-20.
- The global asthma report. Auckland, New Zealand: Global Asthma Network. 2018.
- Deutsches Ärzteblatt Internationa, Dtsch Arztebl Int. 2008; 105: 385-394.
- Gaude GS, Hattiholi J, Chaudhury A. Role of health education and self-action plan in improving the drug compliance in bronchial asthma. J Fam Med Primary Care. 2014; 3: 33-38.
- Wang R and Lin J. Analysis of the Mechanism of Zhichuanling Oral Liquid in Treating Bronchial Asthma Based on Network Pharmacology. Evidence- Based Complementary and Alternative Medicine. 2020; 2020: 10.
- Sodhi R, Prasad R, Kushwaha R, Kant S, Verma SK, Garg R, et al. A study to know the knowledge, attitude, and practices of patients of bronchial asthma. Int J Med Public Health. 2013; 3: 159-162.
- Gajanan G, Padbidri VS, Chaudhury A. Assessment of Knowledge and Attitude of Parents Towards the Allergy and Bronchial Asthma in Their Children. Inter. J. Med. Public Health. 2016; 6: 121-125.
- Suarez-Varela MM, Garcia de Andoin N, Batlles-Garrido J, et al. Geographic variance in the prevalence of asthma symptoms in Spanish children and adolescents.International study of Asthma and Allergies in Childhood (ISSAC) Phase 3, Spain]. Arch Bronchoneumol. 2005; 41: 659-666.
- Anjan kumar DS, Adepu R, Parthasarathi G, Mahesh PA. Impact of community pharmacist provided patient education in asthma patients on treatment outcomes-A study. Indian J Pharm Educ Res. 2009; 43: 125-133.
- M Ichinose, et al. Japanese guidelines for adult asthma. Allergology International. 2017; 66: 163-189.
- Bergeron C, et al. Airway remodelling in asthma: From benchside to clinical practice. Can Respir J. 2010; 17; e85-93.
- Kunzmann S, Schmidt-Weber C, Zingg JM, et al. Connective tissue growth factor expression is regulated by histamine in lung fibroblasts: Potential role of histamine in airway remodeling. J Allergy Clin Immunol. 2007; 119: 1398- 1407.
- Westergren-Thorsson G, Chakir J, Lafreniere-Allard MJ, Boulet LP, Tremblay GM. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol. 2002; 34: 1256-1267.
- Xu P, Wang L, Chen D, Feng M, Lu Y, Chen R, et al. The application of proteomics in the diagnosis and treatment of bronchial asthma. Ann Transl Med. 2020; 8: 132.
- Rhee CK. Nanotechnology as a savior in asthma management. Ann Transl Med. 2019; 7: 517.
- Burgstaller G, Oehrle B, Gerckens M, et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017; 50: 1601805.
- MH Smolensky, et al. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Advanced Drug Delivery Reviews. 2007; 59: 852-882.
- Valero A, Ribó P, Maíz L, et al. Asthma patient satisfaction with different dry powder inhalers. Expert Rev Respir Med. 2019; 13: 133-138.
- Lee Y, Hwang YH, Kim KJ, et al. Proteomic and transcriptomic analysis of lung tissue in OVA-challenged mice. Arch Pharm Res. 2018; 41: 87-100.
- S Ragab, et al. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J. 2006; 28: 68-74.
- Sami Manzoor, et al. To Compare the Efficacy of Doxophylline from Theophylline in Asthma. Journal of Clinical and Diagnostic Research. 2015; 9: FC05-FC08.
- Lazarus SC. Clinical practice. Emergency treatment of asthma. N Engl J Med. 2010; 363: 755- 764.
- Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with metaanalysis. Thorax. 2005; 60: 740-746.
- Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003; 361: 1071- 1076.
- K Maneechotesuwan, S Essilfie-Quaye, S Meah, C Kelly, SA Kharitonov, IM Adcock, et al. Formoterol attenuates neurophilic airway inflammation in asthma, Chest. 2005; 128: 1936-1942.
- Nair P, Milan SJ, Row BH. Addition of intravenous aminophyllineto inhaled beta agonists in adults with acute asthma (Review). Cochrane Database Syst Rev. 2012; 12: 2742.
- Goldstein MF, Chervinsky P. Efficacy and safety of doxophylline compared to theophylline in chronic reversible asthma-a double-blind randomized placebocontrolled multicentre clinic trial. Med SciMonit. 2002; 8: 297-304.
- Campos HS, Xisto DG, Oliveira MB, et al. Protective effects of phosphodiesterase inhibitors on lung function and remodeling in a murine model of chronic asthma. Braz J Med Biol Res. 2006; 39: 283-287.
- Kumar RK, Herbert C, Thomas PS, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther. 2003; 307: 349-355.
- Henderson WR Jr, Tang LO, Chu SJ, et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002; 165: 108-116.
- Muz MH, Deveci F, Bulut Y, Ilhan N, Yekeler H, Turgut T. The effects of low dose leukotriene receptor antagonist therapy on airway remodeling and cysteinyl leukotriene expression in a mouse asthma model. Exp Mol Med. 2006; 38: 109-118.
- Kelly MM, Chakir J, Vethanayagam D, et al. Montelukast treatment attenuates the increase in myofibroblasts following low-dose allergen challenge. Chest. 2006; 130: 741-753.
- S Baddar, B Jayakrishnan and OA Al-Rawas. “Asthma control: importance of compliance and inhaler technique assessments,” Journal of Asthma. 2014; 51: 429-434.
- B Ding and M Small. “Disease burden of mild asthma: findings from a crosssectional real-world survey,” Advances in, erapy. 2017; 34: 1109-1127.
- Buhl R, Berdel D, Criee C-P, Gillissen A, Kardos P, Kroegel C, et al. Leitlinie zur Diagnostik und Therapie von Patienten mit Asthma. Pneumologie. 2006; 60: 139-183.
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Bene fits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005; 60: 309-316.
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007; 101: 1483-1492.