
Case Series
Austin J Pulm Respir Med. 2022; 9(1): 1088.
Pharyngeal Electrical Stimulation Treatment of Critically Ill Intensive Care Tracheostomized Patients Presenting with Severe Neurogenic Dysphagia: A Case Series
Traugott MT*, Hoepler W, Kelani H, Schatzl M, Friese E and Neuhold S
¹Department with Infectious Diseases and Tropical Medicine, Klinik Favoriten, Austria
*Corresponding author: Marianna Theresia Traugott, Fourth Medical Department with Infectious Diseases and Tropical Medicine, Klinik Favoriten, KundratstraΒe 3, 1100 Vienna, Austria
Received: June 16, 2022; Accepted: July 20, 2022; Published: July 26, 2022
Abstract
Purpose: To assess the benefits of Pharyngeal Electrical Stimulation (PES) in critically ill tracheostomized patients with severe neurogenic dysphagia.
Methods: A retrospective outcome analysis of tracheostomized patients weaned from mechanical ventilation and treated with PES in a medical ICU.
Results: Nineteen patients (mean age: 64 years), admitted to the ICU mainly because of severe acute infections, were treated with PES whilst still tracheostomized (mean duration of intubation prior to tracheostomy: 12 days). Following the start of PES, 15/19 patients were successfully decannulated during their hospital stay (mean time to decannulation: 13 days); 11 of the 15 surviving patients experienced a complete restoration of swallowing function. Among patients with available data, the mean time to improved feeding status from “nil by mouth” was three days with thickened fluids and ten days with thin oral fluid. The mean length of stay was eleven days in the ICU and 56 days in the hospital. PES treated tracheostomized patients had a shorter mean LOS in the ICU (47 vs 58 days) and in the hospital (109 vs 125 days) compared to non- PES treated ones. No serious adverse events related to PES treatment were observed, and no patients required recannulation.
Conclusions: In this mixed population, PES led to improved swallowing function resulting in successful decannulation of 15/19 patients and return to normal oral intake at hospital discharge in 11/15 patients with severe neurogenic swallowing disorders and tracheostomy.
Keywords: Neurogenic dysphagia; Swallowing disorders; Intensive and critical care; Pharyngeal electrical stimulation; Tracheostomy; Prolonged mechanical ventilation
Introduction
Swallowing disorders (dysphagia) are associated with an increased risk of delayed decannulation and prolonged Intensive Care Unit (ICU) stay. Outside the ICU-setting, dysphagia can lead to dehydration, malnutrition, and death [1]. The use of analgesic and sedative drugs may also contribute to dysphagia [1]. Critically ill ICU patients often require endotracheal intubation and sometimes tracheostomy after prolonged mechanical ventilation. Following tracheostomy, a dysphagia incidence of 11 to 93% has been reported [2]. Patients with persistent and severe dysphagia fail to be decannulated until hospital discharge, which can contribute to mortality [3]. Nevertheless, in most ICUs, dysphagia screening is not systematically performed [4]. One of the largest studies to date implementing systematic dysphagia screening in the ICU (DYnAMICS), identified post-extubation swallowing disorders at ICU discharge in 10% of cases, and in 60% of dysphagia cases, swallowing disorders persisted until hospital discharge [5]. Behavioral swallowing interventions, which include compensatory strategies, rehabilitative exercises, and manoeuvers, generally have limited scientific evidence and appear to be only moderately effective in patients presenting with severe neurogenic dysphagia [6]; in addition, these interventions require active patient participation. In critically ill ICU patients, active engagement is frequently not possible.
Pharyngeal Electrical Stimulation (PES) is a novel and innovative treatment for restoring neurological control of swallowing [7]. It was first validated in non-ventilated stroke patients with dysphagia [8,9] and later in tracheostomized stroke patients with severe dysphagia [10-12]. A meta-analysis including three RCTs concluded that PES treatment was associated with less aspiration and less dysphagia in patients with poststroke dysphagia [13]. Muhle et al. reported on 23 tracheostomized stroke patients who could not be decannulated due to severe and persisting dysphagia; after PES treatment, decannulation was successful in 79% of patients [11]. In the prospective, single-blinded, randomized PHAST-TRAC trial, PES enabled earlier decannulation of tracheostomized patients presenting with severe neurogenic dysphagia after stroke [10]. In the prospective single-arm observational cohort PHADER study, 245 patients with neurogenic dysphagia following stroke or traumatic brain injury, with and without mechanical ventilation and tracheostomy, as well as non-stroke ventilated patients, were treated with PES showing a significant improvement in airway safety and diet advancement [14]. Moreover, PES has been shown to be effective and safe in the treatment of dysphagia associated with several other pathologies: Guillain-Barré syndrome [15], multiple sclerosis [16], COVID-19 infection [17,18] and critical illness polyneuropathy [19].
In this retrospective outcome analysis study, we aimed to determine the benefits of PES treatment in critically ill, tracheostomized patients from an internal medicine ICU with severe swallowing impairment following prolonged mechanical ventilation.
Methods
Patients
This case series describes 19 critically ill ICU patients with severe neurogenic dysphagia following prolonged mechanical ventilation who were treated with PES while tracheostomized in a public hospital in Vienna (Austria) between October 2017 and January 2020. A retrospective analysis of the patients’ health data was performed to gain improved understanding of PES treatment outcomes. Disease severity at ICU admission was categorized using the APACHE II disease severity score [20,21]. Patients underwent dysphagia screening by nursing staff and clinical swallowing evaluations by a Speech and Language Therapist (SLT) in the ICU. Patients were followed-up until hospital discharge for critical outcomes related to dysphagia (tracheostomy decannulation, swallowing and feeding status) and general outcome parameters (Length Of Stay (LOS) in ICU and hospital, discharge destination, and mortality). Data on the occurrence of device deficiencies and serious adverse events related to PES-treatment or the device were also collected. Feeding status was evaluated using functional swallowing outcome measures including the Dysphagia Severity Rating Scale (DSRS, 12 being the worst score) [22] and Functional Oral Intake Scale (FOIS, 1 being the worst score) [23].
PES Treatment
PES treatments were applied using the commercially available Phagenyx® system (Phagenesis Ltd, Manchester, UK). This medical device received CE certification in 2012 and consists of a base station and a specially designed, single-patient use nasogastric catheter with built-in electrodes. The electrodes of the PES-catheter are positioned in a specific location within the pharynx and used to deliver trains (200 μs pulses at 5 Hz) of pharyngeal electrical simulation for ten minutes per day for three consecutive days (=one cycle) and repeated in a second cycle of three consecutive treatment days if required. The current intensity (mA) of PES is optimized to the patient based upon their individualized sensory capacity prior to every treatment session, as reported previously [10,19] The Phagenyx® catheter can also be used as a nasogastric tube for enteral feeding.
Data collection and Statistical Analysis
The following data were extracted from available medical records, anonymized, and analyzed: i) patients’ demographics and clinical characteristics (age, gender, disease severity, diagnoses, comorbidities, cause of death) at ICU admission; ii) duration of intubation, sedation, and time from intubation until tracheostomy; iii) dysphagia assessments; iv) PES treatment parameters (threshold, tolerance, and stimulation intensities in mA); and v) PES treatment outcomes (decannulation status, as well as swallowing and feeding status).
Ethical Considerations
This retrospective case series analysis was conducted in accordance with the principles of Good Clinical Practice and following the Declaration of Helsinki. Study participants’ privacy and confidentiality was guaranteed according to Austrian law (Austrian Data Protection Act, version: 25 May 2018; BGBl. I Nr. 165/1999).
Statistical Analysis
All anonymized data were centrally collected and analyzed at our medical department using descriptive statistics. Descriptive and categorical data are expressed as frequencies and proportions, while continuous data are reported as mean and range.
Results
Description of Cases
A total of 19 critically ill, internal medicine patients admitted to ICU were treated with PES for severe neurogenic dysphagia following prolonged endotracheal intubation and subsequent tracheostomy between October 2017 and January 2020. Baseline patient characteristics are described in (Table 1). Overall, patients were predominantly male (68%), with a mean age of 64 years (range: 44-81), 63% being 61 years old or older. At ICU admission, the mean disease severity score (APACHE II) was 20 (range: 4-29), with ten out of 19 patients (53%) presenting with a high mortality probability (APACHE II score: 20-29). Most frequent reasons for ICU admission were acute infections (n=17, 89%), pneumonia (n=9, 47%), sepsis or septic shock (n=7, 37%), acute renal failure (n=6, 32%), and acute exacerbation of chronic obstructive pulmonary disease (COPD) (n=6, 32%). Some patients were admitted with several of the above cited diagnoses. All patients presented with comorbidities; the most frequent were hypertension (n=15, 79%), diabetes mellitus type II (n=9, 47%), nicotine abuse (n=8, 42%), COPD (n=7, 37%), coronary heart diseases (n=7, 37%), infectious diseases (n=6, 32%), chronic renal insufficiency (n=5, 26%), hypothyroidism (n=5, 26%), hyperlipidemia (n=5, 26%), chronic respiratory insufficiency (n=3, 16%) or obesity (n=2, 11%).
Mean variables (N=19)
Gender, n (%)
Male
13 (68)
Female
6 (32)
Age, years (range)
64 (44-81)
Age groups (years), n (%)
41-50
2 (11)
51-60
5 (26)
61-70
4 (21)
71-80
7 (37)
81-90
1 (5)
Disease severity (APACHE IIb score) at ICUc admission
Severity score, score (range up to 71)
20 (4-29)
Mortality probability, n (%)
Low (APACHE II score 0-9)
1 (5)
Moderate (APACHE II score 10-19)
8 (42)
High (APACHE II score 20-29)
10 (53)
ICU stay, n (%)
Diagnosis at admission
Infectious diseases (ARDSd; influenza A pneumonia; HIVe; cerebral toxoplasmosis; West-Nil encephalitis; MRSAf; MRGNg)
17 (89)
Pneumonia
9 (47)
Sepsis or septic shock
7 (37)
Acute renal failure
6 (32)
COPDh
6 (32)
Polyneuropathy
3 (16)
Cardiac arrest
2 (11)
Herpes Zoster
2 (11)
Othersi
6 (32)
Most frequent or relevant medical history/comorbidities
Hypertension
15 (79)
Diabetes mellitus type II
9 (47)
Nicotine abuse
8 (42)
COPDh
7 (37)
Coronary heart diseases
7 (37)
Infectious diseases (HIVe, hepatitis B/C, tuberculosis)
6 (32)
Chronic renal insufficiency
5 (26)
Hypothyreosis
5 (26)
Hyperlipidemia
5 (26)
Chronic respiratory insufficiency
3 (16)
Obesity
2 (11)
Time between onset of symptoms and ICU admission, days (range)
4 (1-21)
Invasive mechanical ventilation, days (range)
Duration of endotracheal intubation
12 (6-22)
Duration of tracheostomy cannulation
30 (12-63)
Sedation
Duration of sedation
18 (1-47)
High sedation level (RASSj -4/-5), n (%)
16 (84)
Dysphagia Severitykpre-PES treatment
Patients with worst DSRSl score pre-PES, n (%)
19 (100)
Patients with worst FOISm score pre-PES, n (%)
19 (100)
Notes: N, overall population considered; aPercentages may not equal to 100 because of rounding; bAPACHE, acute physiology and chronic health evaluation; cICU, intensive care unit; dARDS, acute respiratory distress syndrome; eHIV, human immunodeficiency virus; fMRSA,methicillin-resistantstaphylococcus aureus;gMRGN, multidrug resistant Gram-negative bacteria; hCOPD, chronic obstructive pulmonary disease; iOthers included epilepsy, opiate overdose, pericardial effusion, breast cancer relapse and sacral decubitus ulcer, and concerned one patient each; jRASS, Richmond Agitation-Sedation Scale; kOnly clinical bedside examination performed; lDSRS (Dysphagia Severity Rating Scale) assessment, worst DSRS=12; mFOIS (Functional Oral Intake Scale) assessment, worst FOIS=1
Table 1: Baseline demographics and clinical characteristicsa.
The mean duration between onset of symptoms and ICU admission was four days (range: 1-21). The mean duration of endotracheal intubation was twelve days (range: 6-22). On average, patients were treated with sedatives for the first 18 days (range: 1-47) in the ICU. In total, 16 patients (84%) were initially deeply sedated with a Richmond Agitation-Sedation Scale (RASS) score of -4 to -5. All intubated patients received tracheostomy because of continuous need for mechanical ventilation and airway protection. The mean duration of cannulation was 30 days (range: 12-63).
Dysphagia Assessment Pre-PES
Secretion management and readiness for decannulation was assessed by nursing staff, Speech and Language Therapists (SLTs) and physicians. The clinical swallowing evaluations were performed by SLTs including the blue dye swallow test. Instrumental assessments such as Flexible Endoscopic Evaluation of Swallowing (FEES) or Video Fluoroscopy (VFS) were not routinely performed at the ICU. All patients in this cohort presented with suspected severe neurogenic dysphagia at baseline characterized by inability to manage secretions with no swallowing attempts and weak or ineffective cough; therefore, “nil by mouth” (translating into a Dysphagia Severity Rating Scale (DSRS) of 12 out of 12, and a Functional Oral Intake Scale (FOIS) of 1 out of 7) was recommended by SLTs (Table 1).
PES Treatment
In addition to standard of care therapy performed by SLTs, all 19 patients received individually optimized PES. PES characteristics are reported in Table 2A. On average, patients started PES treatment 34 days (range: 21-71) after ICU admission and 18 days (range: 3-39) post tracheostomy. The first PES session was performed on average nine days (range: 0-18) after the baseline swallowing assessment. Patients received on average four PES sessions (range: 2-7) over a mean of four days (range: 2-14) with each session lasting ten minutes (range: 8-10). Swallowing improvement was regularly assessed by SLTs after PES sessions. This information was used to determine if additional PES sessions were needed. This was the case for two patients, who received two consecutive cycles of PES, with a mean interval of twelve days. Among all treated patients, the mean sensory threshold was 17 mA (range: 7-26), the mean tolerance threshold was 29 mA (range: 13-41), and the mean optimized stimulation intensity was 25 mA (range: 10-37). Patients who received more than three PES sessions had lower mean threshold-, stimulation-, and tolerance levels than those who received three or less PES sessions (Figure 1).
(N=19)
Mean (range)
Time between ICU admission and first PES session, days
34 (21-71)
Time between extubation and first PES, days
18 (3-39)
Time between swallowing assessment and first PES session, days
9 (0-18)
Number of PES sessions, n
4 (2-7)
Time between first and last PES sessions, days
4 (2-14)
Duration of each PES session, min
10 (8-10)
Stimulation, mA
25 (10-37)
Threshold, mA
17 (7-26)
Tolerance, mA
29 (13-41)
at first session
34 (12-50)
at last session
24 (10-45)
Notes: overall population considered; PES, Pharyngeal Electrical Stimulation; mA, milliampere.
Table 2A: Pharyngeal Electrical Stimulation (PES) characteristics.
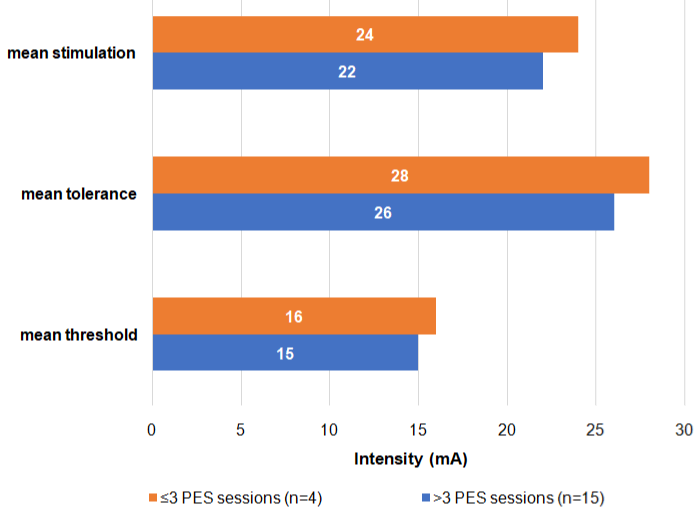
Figure 1: Mean stimulation, tolerance, and threshold intensities of PES therapy (N=19).
PES Treatment Outcome
Decannulation: Decannulation of tracheostomized patients is only safe if the respiratory effort is low and if patients can swallow their saliva independently, which is essential to avoid aspiration pneumonia. Table 2B presents the outcomes of PES therapy. Overall, 15 out of 19 patients (79%) were successfully decannulated during their hospital stay. Four patients remained cannulated and died either in the ICU or during the hospital stay. The mean time to decannulation after the initiation of PES treatment was 13 days (range: 2-43); nine out of 17 patients (53%) were already decannulated within ten days after the first PES session, 12 out of 17 patients (71%) were decannulated within 15 days after the first PES session (Figure 2). Most patients (10/15, 67%) were decannulated during their PES treatment cycle(s). Five patients out of 15 (33%) were still cannulated during their final PES session and were decannulated within a mean of seven days (range: 0-17) post-treatment.
Mean variables (N=19)a
PES, days (range)a
Time from first PES session to decannulation (N=17)b
13 (2-43)
Time from last PES session to decannulation (N=7)c
7 (0-17)
Number of patients still tracheostomized at last PES session, n (%)
7 (33)
Swallowing improvement
Patients with highest DSRS scored at discharge to home or nursing home, n (%)
11 (58)
Dysphagic at discharge, n (%)
From ICU (N=17)e
7 (41)
From hospital (N=15)f
1 (7)
Feeding improvement, days (range)a
Time with PEG-tube or nasogastric tube (N=14)g,h
58 (26-85)
Time between last PES session and PEG-tube or nasogastric tube removal (N=14)g,h
17 (1-54)
Time to thickened oral fluids post-PES, days (range), (N=10)I
3 (1-11)
Time to normal oral fluids post-PES, days (range), (N=10)i
10 (2-32)
Time to modified diet post-PES, days (range), (N=15)i
6 (0-41)
Time to normal diet post-PES, days (range), (N=10)i
14 (4-32)
Patients with highest FOIS scorej at discharge to home or nursing home, n (%)
11 (58)
Length of stay post-PES, days (range)
At ICU (N=17)k
11 (2-50)
At hospital (N=15)l
56 (6-179)
All-cause mortality, n (%)
At ICU
2 (11)
At hospital
4 (21)
Notes: aExcept other indicated; N, overall population considered for the analysis; bOne patient was decannulated two days before PES start and one patient died before being decannulated; cTwelve patients were decannulated before last PES session; dDSRS (Dysphagia Severity Rating Scale) assessment among the 15 discharged patients, with highest score of 0 indicating normal fluids and a solid diet;eTwo patients died during ICU stay; fFour patients died during hospital stay; gExcept three patients who were still dependent of gastrostomy or nasogastric tube when they died during hospital stay;hExcept two patients who were discharged to home or nursing home with gastrostomy tube; iCalculated fromavailable data; jFOIS (Functional Oral Intake Scale) assessment among the 15 discharged patients, with highest score of 7 indicating a normal functional oral intake; kExcept two patients who died during ICU stay; lExcept four patients who died during hospital stay; ICU, intensive care unit; PES, Pharyngeal Electrical Stimulation.
Table 2B: Therapy outcomes.
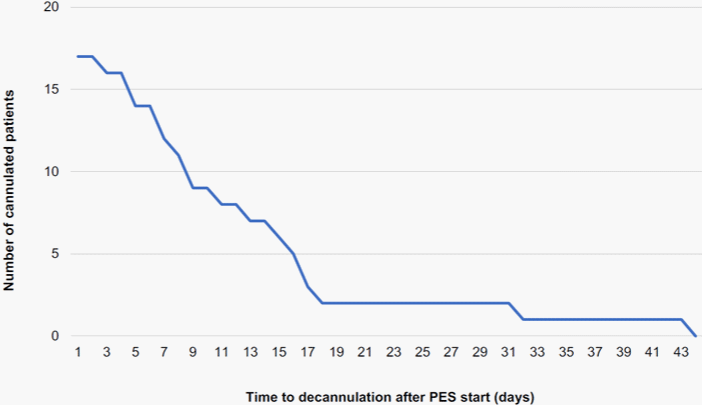
Figure 2: Kaplan-Meier curve of time to decannulation after PES therapy start (N=17).
Oral intake outcome: Among the 15 surviving patients, 11 (73%) experienced a complete restoration of swallowing function and returned to normal oral intake (DSRS=0 and FOIS=7, indicating an optimal management of thin fluids and regular diet without supervision) at hospital discharge. The four remaining surviving cases had a variable but not full improvement of swallowing function; two patients left the hospital against medical advice after swallowing improved and were lost to follow-up; one patient with a mental health disorder still experienced dysphagia upon his transfer to a nursing home after 271 days in the hospital; one patient was no longer dysphagic, but received supplemental enteral nutrition and hydration via a PEG-tube (percutaneous endoscopic gastrostomy) for three additional months following hospital discharge due to being underweight. In the 15 patients with complete swallowing restoration during the hospital stay, the mean time from first oral intake to thickened fluids was three days (range: 1-11), while the mean time to oral intake of thin fluids was ten days (range: 2-32). Intake of a modified dietary texture (pureed) could be started on average six days (range: 0-41) post-PES and normal diet was resumed 14 days (range: 4-32) after the last PES session.
Length of ICU/hospital stay: Two patients died during their ICU stay. Looking at the 17 remaining patients discharged from the ICU, the mean ICU LOS post-PES therapy was eleven days (range: 2-50). In total, eleven out of 17 patients (65%) were discharged from the ICU within ten days post-PES and 14 out of 17 patients (82%) within 15 days post-PES (Figure 3). All patients discharged from the ICU were transferred to another ward for further inpatient rehabilitation. In most cases this was due to ICU-acquired weakness, a common complication following ICU survival [24]. Subsequently, patients were discharged home (n=10, 67%) or transferred to another rehabilitation center (n=3, 20%) or nursing home (n=2, 13%). Mean hospital LOS post-PES was 56 days (range: 6-179) (Table 2B). Six out of 15 patients (40%) were discharged from hospital within 25 days post-PES and nine out of 15 patients (60%) within 50 days after the last PES session (Figure 4). Seven patients (41%) experienced ongoing dysphagia at ICU discharge, while only one of these patients (7%) still presented with swallowing difficulty at hospital discharge (Table 2B). In total, the nasogastric or PEG-tube could be removed in 13 out of 15 (87%) patients before their discharge from hospital; however, one patient needed his PEG-tube for 98 additional days at home as he was underweight requiring high caloric supplement. This high caloric treatment was independent from dysphagia; the other patient had a mental health disorder and was still experiencing dysphagia upon his transfer to a nursing home after 250 days in hospital. Without these outliers, mean duration with a PEG or nasogastric tube was 58 days (range: 26-85).
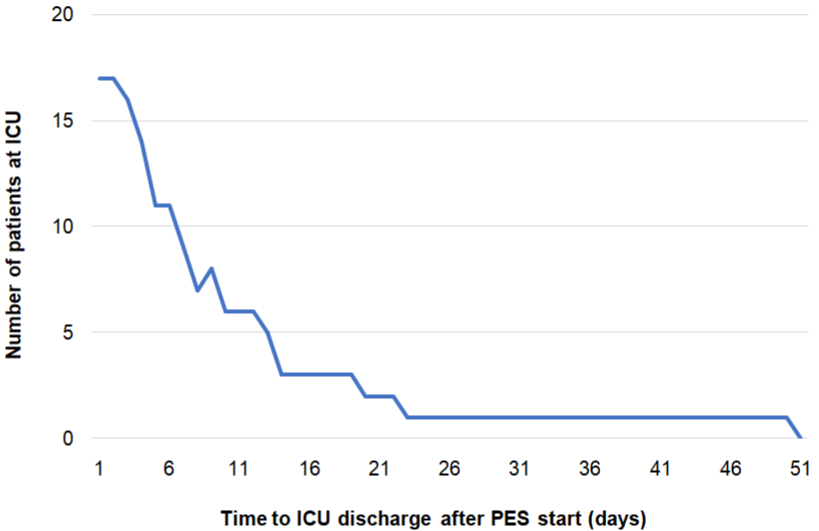
Figure 3: Kaplan-Meier curve of time to ICU discharge post-PES (N=17).
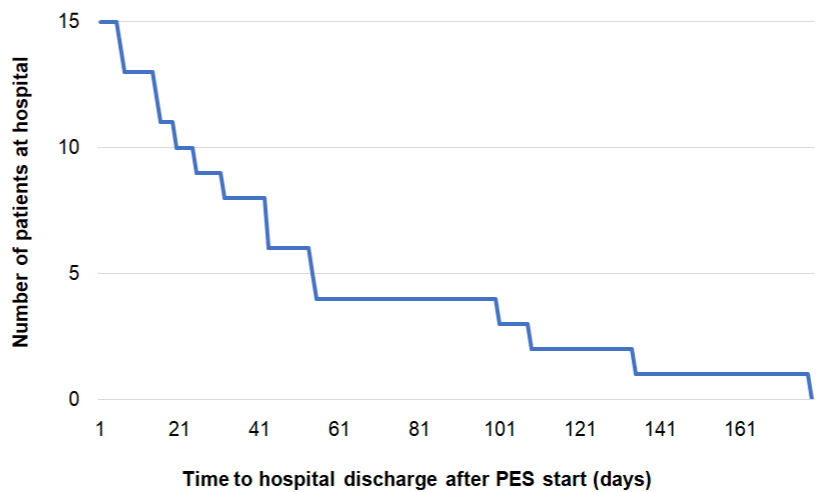
Figure 4: Kaplan-Meier curve of time to hospital discharge post-PES (N=15).
Additionally, we analyzed hospital-discharged tracheostomized PES-treated (n=15) versus non-PES-treated patients (n=16) who had been admitted to our ICU during the same time period from August 2017 to February 2020; we found that PES-treated tracheostomized patients had a shorter mean LOS in the ICU (47 vs 58 days) and in the hospital (109 vs 125 days) compared to non-PES treated ones.
Hospital Complications: In total, 17 out of 19 patients (89%) experienced complications during the ICU stay or thereafter. The most frequent complication was pneumonia (ventilator-associated pneumonia in 16 cases and aspiration pneumonia in 1 case); pneumonias occurred mainly prior to PES treatment. Two patients died in the ICU: One died due to decompensated cardiopathy 48 days after the last PES session and the other following deterioration of his general health status due to pneumonia nine days after the last PES session. Two additional patients died after discharge from the ICU(23- and 49-days after PES treatment, respectively). All deaths are considered unrelated to PES therapy.
Following PES treatment, 15 out of 19 patients (79%) were successfully decannulated with no need for recannulation. No PES treatment or device-related serious adverse events were observed in any of the patients. One patient reported a slight transient pain during the initial phase of PES parameter optimization, which may have been caused by nausea, but this did not preclude treatments being delivered at a lower stimulation intensity (8 mA). Additionally, one device deficiency (connecting cable failure) occurred, causing treatment delay in one patient, which was resumed as soon as the cable was replaced.
Discussion
Severely ill ICU-patients who require tracheostomy due to prolonged intubation and mechanical ventilation are at great risk of developing dysphagia. Dysphagic patients with lengthy ICU stays also demonstrate slower recovery mainly due to dysphagia-related complications, such as aspiration-induced pneumonia, longer tube feeding and higher probability for ICU-acquired infections, such as central venous catheter infections [5]. Moreover, duration of mechanical ventilation and dysphagia status are shown to be significantly associated with a delayed return to normal oral intake, longer hospitalization as well as increased morbidity and mortality rates [5].
At ICU admission, more than half of our patients presented with a high risk of death (10 out of 19 with APACHE II score: 20-29). Most patients were middle-aged (mean age: 64 years), had multiple known comorbidities and the majority had experienced additional hospitalacquired infections. Our patients experienced dysphagia after a mean intubation duration of twelve days and a mean time since tracheostomy of 30 days. Moreover, analgesic and sedative drugs are considered to be a major contributor to dysphagia [5]. Our analyzed population had been highly sedated for 18 days on average.
Following PES, 15 out of 19 (79%) patients could be decannulated. 14 out of 15 (93%) discharged patients left hospital without any swallowing disorder. This outcome is remarkable, in comparison to a study that found persistent swallowing difficulty at hospital discharge in 60% of post extubation dysphagia cases [5]. Previously published data in dysphagic stroke patients reported a decannulation rate of 73% and 75% after PES treatment [11, 12]. Overall, this data demonstrates the efficacy of PES in both neurological (stroke) and non-neurological dysphagic patients.
All reported cases initially presented with severe swallowing dysfunction resulting in a “nil by mouth” order at baseline just prior to PES treatment initiation. In total, 11 out of 15 decannulated patients showed recovered swallowing function with return to a normal diet and independent feeding following PES therapy at hospital discharge. Additionally, 87% of patients who survived had their nasogastric or PEG tube removed before hospital discharge. This is in strong contrast to previous data, reporting a rate of approximately 20% of PEG tubes that could be removed before the patients’ hospital discharge [25].
Our results are in line with other studies: A study with tracheostomized patients with severe post-stroke dysphagia showed decannulation rates of 49% in the PES group versus only 9% in the sham group [10]. Another randomized controlled trial found decannulation rates of 75% in the PES group versus 20% in the control group [12]. A meta-analysis including 22 studies and 1,700 mainly stroke patients showed that swallowing treatments, including sensory electrical stimulation, during intubation or postextubation resulted in a reduced risk for pneumonia [26]. However, swallowing disorders observed after a stroke have a different etiology than ventilator-acquired dysphagia, and therefore other mechanisms might be involved in recovery [27]. Dysphagia causes are certainly multifactorial, including oropharyngeal and/or laryngeal desensitization because of the endotracheal tube, neurological impairment, critical illness neuropathy, sedating medications and an altered level of consciousness [28, 29].
Impaired sensory feedback seems to be the key driver of ICUrelated dysphagia. PES stimulation levels are personalized prior to each treatment session for optimal stimulation intensity. The stimulation thresholds of our patients declined in the course of the treatment, because patients became more sensible to the electrical stimulation: the median tolerance levels decreased from 35 mA to 24 mA. This enhanced sensibility could be interpreted as further proof of peripheral nervous system restoration.
The higher mean stimulation levels in patients who only received three or less PES treatment can be explained by the higher stimulation intensities in the first treatment sessions. Consequently, patients with more treatment sessions had lower median stimulation levels.
PES therapy appeared to have a positive impact on long-term swallowing function, as none of the 15 patients that had been successfully decannulated after PES required recannulation. One patient was still cannulated at the time of his transfer to a nursing home, this was independent of dysphagia. Another patient was still dysphagic at hospital discharge. Overall, four patients died in the ICU or subsequent hospital unit because of a newly acquired infection or their underlying comorbidities.
Dysphagia prolongs the hospital length of stay by about 40% [30]. We retrospectively compared all tracheostomized patients in our ICU who received or did not receive PES treatment in the same time period. PES treatment of tracheostomized patients was associated with a shorter mean LOS in the ICU and in the hospital (11- and 16- days LOS reductions, respectively).
PES treatment was started relatively late, on average nine days (range: 0-18) after the baseline swallowing assessment. This treatment delay can be explained by two factor. Firstly, the limited availability of the treatment device, which is shared with the neurology department. Secondly, PES treatment had not yet been fully established in our ICU when the first patients in this case series were treated.
This analysis has several limitations, including the small size of our dysphagic ICU population, the observational design, the lack of a dysphagic matched control group which was not PES-treated, as well as the lack of instrumental assessment procedures due to logistic and feasibility reasons at our ICU. Moreover, we want to emphasize that these outcomes are depicting the real-world nature of our ICU setting with critically ill patients having fluctuating medical conditions. However, to our knowledge, this case series is the first one to review the use of PES for the treatment of severe dysphagia within a nonneurological, critically ill tracheostomized patient population in an internal medicine ICU setting.
Conclusions
In our case series, including 19 critically ill tracheostomized ICU patients, PES therapy led to improved swallowing function enabling decannulation, return to safe oral intake as well as faster ICU and hospital discharge in most patients.
Ethics Approval and Patient’s Informed Consent
According to Austrian laws, ethics commission approval or patient consent was not necessary for this retrospective analysis.
Availability of Data and Materials
Anonymized source data is available upon request from the corresponding author.
Declarations of Interest
None of the authors have competing interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors would like to thank Florence Boulmé, PhD, for writing assistance.
References
- Burgos R, Bretón I, Cereda E, Desport JC, Dziewas R, Genton L, et al. ESPEN guideline clinical nutrition in neurology. Clinical nutrition. 2018; 37: 354-396.
- Skoretz SA, Anger N, Wellman L, Takai O, Empey A. A Systematic Review of Tracheostomy Modifications and Swallowing in Adults. Dysphagia. 2020; 35: 935-947.
- Martinez GH, Fernandez R, Casado MS, Cuena R, Lopez-Reina P, Zamora S, et al. Tracheostomy tube in place at intensive care unit discharge is associated with increased ward mortality. Respiratory care. 2009; 54: 1644- 52.
- Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Critical Care. 2019; 23.
- Schefold JC, Berger D, Zürcher P, Lensch M, Perren A, Jakob SM, et al. Dysphagia in Mechanically Ventilated ICU Patients (DYnAMICS): A Prospective Observational Trial. Critical Care Medicine. 2017; 45: 2061-2069.
- Bath PM, Lee HS, Everton LF. Swallowing Therapy for Dysphagia in Acute and Subacute Stroke. Stroke. 2019; 50.
- Phagenesis, https://www.phagenesis.com/news/; 2022.
- Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, et al. Driving Plasticity in Human Adult Motor Cortex Is Associated with Improved Motor Function after Brain Injury. Neuron. 2002; 34: 831-840.
- Jayasekeran V, Singh S, Tyrrell P, Michou E, Jefferson S, Mistry S, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010; 138: 1737-1746.e2.
- Dziewas R, Stellato R, Tweel IVD, Walther E, Werner CJ, Braun T, PHASTTRAC investigators. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHASTTRAC): a prospective, single-blinded, randomised trial. The Lancet Neurology. 2018; 17: 849-859.
- Muhle P, Suntrup-Krueger S, Bittner S, Ruck T, Claus I, Marian T, et al. Increase of Substance P Concentration in Saliva after Pharyngeal Electrical Stimulation in Severely Dysphagic Stroke Patients - an Indicator of Decannulation Success? Neurosignals. 2017; 25: 74-87.
- Suntrup S, Marian T, Schröder JB, Suttrup I, Muhle P, Oelenberg S, et al. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: a randomized controlled trial. Intensive Care Medicine. 2015; 41: 1629-1637.
- Scutt P, Lee HS, Hamdy S, Bath PM. Pharyngeal Electrical Stimulation for Treatment of Poststroke Dysphagia: Individual Patient Data Meta-Analysis of Randomised Controlled Trials. Stroke Research and Treatment. 2015; 2015: 1-8.
- Bath PM, Woodhouse LJ, Suntrup-Krueger S, Likar R, Koestenberger M, Warusevitane A, et al. Pharyngeal electrical stimulation for neurogenic dysphagia following stroke, traumatic brain injury or other causes: Main results from the PHADER cohort study. EClinicalMedicine. 2020; 28: 100608.
- Beirer S, Grisold W, Dreisbach J. Therapy-resistant dysphagia successfully treated using pharyngeal electrical stimulation in a patient with the pharyngealcervical- brachial variant of the Guillain-Barré syndrome. eNeurologicalSci. 2020; 20: 100255.
- Restivo DA, Casabona A, Centonze D, Marchese-Ragona R, Maimone D, Pavone A. Pharyngeal Electrical Stimulation for Dysphagia Associated with Multiple Sclerosis: A Pilot Study. Brain Stimulation. 2013; 6: 418-423.
- Traugott M, Hoepler W, Kitzberger R, Pavlata S, Seitz T, Baumgartner S, et al. Successful treatment of intubation-induced severe neurogenic postextubation dysphagia using pharyngeal electrical stimulation in a COVID-19 survivor: a case report. Journal of Medical Case Reports. 2021; 15.
- Blakemore C, Hunter J, Bhasker B. Rapid swallow improvement following pharyngeal electrical stimulation in a COVID-19 patient with long-term severe neurogenic dysphagia: a case report. JRM-CC. 2021; 4: 100073.
- Koestenberger M, Neuwersch S, Hoefner E, Breschan C, Weissmann H, Stettner H, et al. A Pilot Study of Pharyngeal Electrical Stimulation for Orally Intubated ICU Patients with Dysphagia. Neurocritical Care. 2019; 32: 532- 538.
- Jacobs S, Chang RW, Lee B. One year’s experience with the APACHE II severity of disease classification system in a general intensive care unit. Anaesthesia. 1987; 42: 738-44.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13: 818-29.
- Everton LF, Benfield JK, Hedstrom A, Wilkinson G, Michou E, England TJ, et al. Psychometric assessment and validation of the dysphagia severity rating scale in stroke patients. Scientific Reports. 2020; 10.
- Crary MA, Mann GDC, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of physical medicine and rehabilitation. 2005; 86: 1516-1520.
- Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. The New England journal of medicine. 2014; 370: 1626-1635.
- Campos-Martín C, García-Torres MD, Castillo-Martín C, Domínguez- Rabadán R, Rabat-Restrepo JM. Patients Discharged with Home Enteral Nutrition from a Third-Level Hospital in 2018. Nutrients. 2019; 11: 2570.
- Duncan S, McAuley DF, Walshe M, McGaughey J, Anand R, Fallis R, et al. Interventions for oropharyngeal dysphagia in acute and critical care: a systematic review and meta-analysis. Intensive Care Medicine. 2020; 46: 1326-1338.
- Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998; 115: 1104- 1112.
- Brodsky MB, Gilbert RJ. The Long-Term Effects of COVID-19 on Dysphagia Evaluation and Treatment. Archives of Physical Medicine and Rehabilitation. 2020; 101: 1662-1664.
- Ponfick M, Linden R, Nowak DA. Dysphagia--a common, transient symptom in critical illness polyneuropathy: a fiberoptic endoscopic evaluation of swallowing study. Crit Care Med. 2015; 43: 365-72.
- Altman KW, Yu G, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Archives of otolaryngology--head & neck surgery. 2010; 136: 784.