
Clinical Study
Austin J Pulm Respir Med. 2022; 9(2): 1092.
Risk Factors of Pulmonary Embolism in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Retrospective Clinical Study
Peng Ling1,2, Wang An3, Miao YaFang3, Xue Fei3 and Zhou Chao2*
¹Department of Respiratory Medicine, Qiannan Buyi and Miao Autonomous Prefecture People’s Hospital Guizhou, China
²Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area Shanghai, China
³Department of Respiratory Medicine, Zhoupu Hospital Affiliated to Shanghai University of Medicine and Health Sciences, China
*Corresponding author: Chao Zhou, Department of Respiratory Medicine, Guangming Traditional Chinese Medicine Hospital of Pudong New Area Shanghai, No. 43 DongMen Street. Pudong New District, Shanghai 201399, China
Received: September 16, 2022; Accepted: October 19, 2022; Published: October 26, 2022
Abstract
Background: The occurrence of Pulmonary Thromboembolism (PTE) in patients with Acute Exacerbation Of Chronic Obstructive Pulmonary Disease (AECOPD) is not rare, which would seriously affect the prognosis and cause high mortality of patients.
Objective: To investigate the prevalence, risk factors, and clinical characteristics of AECOPD patients with Pulmonary Embolism (PE) complications in a tertiary care center, aiming to reduce the rate of missed diagnosis of PE in patients with AECOPD.
Materials and Methods: We performed a retrospective analysis of patients admitted to our hospital with the first diagnosis of AECOPD from January 2015 to November 2019. Patients were divided into AECOPD and AECOPD +PE groups according to whether or not they had PE complications. The clinical data of the two groups were compared and multiple regression analysis was used to explore the risk factors.
Results: From January 2015 to November 2019, a total of 636 AECOPD patients (aged 76.60 ± 8.38 years, 529 males) were enrolled in this study. Of them, 7.4% (47/636) were diagnosed with PE. Clinical features including age, chest pain, dyspnea, hemoptysis, syncope, Electrocardiogram (ECG), mMRC score, annual acute exacerbation times, history of thrombus, history of surgery within 6 weeks, prolonged immobility ≥3 days, wet rales, pleural effusion, asymmetrical lower extremity edema, history of stroke, pulmonary heart disease, pulmonary encephalopathy, hospitalization days, GOLD grade, total duration, PH, PaCO2, the level of plasma D-dimer and N-terminal pro-brain natriuretic peptide (NT-proBNP) were statistically significant between the two groups (P <0.05). Considering patients with PE as the dependent variables and statistically significant risk factors in the univariate analysis as independent variables, the logistic model analysis was performed. The results indicated that chest pain, syncope, premature contractions, prolonged immobility ≥3 days, history of stroke, pulmonary heart disease, pulmonary encephalopathy, hospitalization days, D-dimer levels, and acute exacerbation times were independent risk factors for AECOPD complicated with PE (P <0.05).
Conclusion: Patients hospitalized for AECOPD should have multi-slice spiral Computed Tomography Pulmonary Angiography (CTPA) to determine whether they present PE complications as soon as possible when combined with chest pain, pulmonary heart disease, prolonged immobility ≥3 days, plasma D-dimer levels higher, and the times of acute exacerbations has increased significantly in the last year.
Keywords: Acute exacerbation; Chronic obstructive pulmonary disease; Pulmonary embolism; Risk factors; D-dimer
Introduction
The prevalence of patients with Chronic Obstructive Pulmonary Disease (COPD) is constantly increasing and is 13.7% in patients under 40 years old and can reach 27% in patients of the elderly (60 years old); currently, the total number of patients with COPD is around 100 million with a double proportion for men (2.2 times the number of women) [1]. COPD is one of the most public chronic diseases such as hypertension and diabetes, and its consequences are fatal for the health of patients, with a heavy socio-economic burden. COPD is considered a stand-alone risk factor for Pulmonary Embolism (PE), an extremely fatal disease) [2] and the prevalence of PE in individuals with COPD is quadrupled in non-COPD patients [3]. The occurrence of PE and Venous Thromboembolism (VTE) in patients with AECOPD was reported to be 19.9% and 29%, respectively [4,5], but according to autopsy data, the prevalence of PE in COPD patients ranges from 28 to 51% [6]. However, due to the presence of non-specific symptoms such as chest pain, hemoptysis, and dyspnea, the clinical symptoms of PE are similar to the deterioration of COPD, which makes it easy to be ignored in patients with AECOPD and lead to poor prognosis.
At present, PE is mainly diagnosed by biomarkers (such as fibrin degradation products D-dimers), echocardiography, pulmonary angiography, and so on. Although plasma D-dimers are widely used in patients with clinical suspicion of VTE with high sensitivity, their specificity is still low, leading to missed or blind further CTPA in some patients. However, CTPA requires the use of intravenous contrast agents, which cannot be used in renal insufficiency patients or antagonistic responses to contrast agents. Therefore, our study is based on the basic information of patients who were admitted to our hospital because of AECOPD in the past five years, to explore the risk factors of AECOPD combined with PE in order to improve clinicians’ understanding of PE, enable timely diagnosis of PE and reasonable treatment in AECOPD patients with PE complications and reduce the risk of death of patients with AECOPD.
Materials and Methods
Inclusion and grouping of participants
Through searching the electronic medical records system, we collected and organized the clinical information of patients admitted to the Department of Respiratory Medicine, Zhoupu Hospital, Pudong New District, Shanghai, China, from January 2015 to November 2019 with AECOPD as the first diagnosis.
Inclusion and exclusion criteria: all included patients were clinically diagnosed with COPD according to the global chronic obstructive pulmonary disease initiative (GOLD) criteria stipulating that after bronchodilator, the forced expiratory volume in one second (FEV1)/Forced Vital Capacity (FVC) is lower than 70% (FEV1/FVC<70%) [7]. Active tuberculosis, pulmonary fibrosis, or bronchiectas is patients were excluded from this study. Obstructive sleep apnea-hypopnea syndrome and asthma patients were included in this study.
The study was approved by the Research Ethics Committee of Zhoupu Hospital affiliated to Shanghai University of Medicine and Health Sciences, Pudong New District, Shanghai, China.
Research Methods
Patients hospitalized for AECOPD were distributed into a group of patients with AECOPD and a group of AECOPD complicated with PE. The clinical characteristics of the two groups, including general conditions (gender, age, pulse), symptoms (dyspnea, cough, sputum, dyspnea, chest pain, hemoptysis, syncope, etc.), signs (asymmetric lower extremity edema, pleura effusion, wet rales), concomitant diseases (hypertension, diabetes, coronary heart disease, malignant tumor), laboratory tests (arterial blood gas analysis, C-reactive protein (CRP), D-dimer, fibrinogen, NT-proBNP, etc.), history of surgery within 6 weeks, prolonged immobility ≥3 days, home oxygen therapy, electrocardiogram, history of stroke, pulmonary encephalopathy, pulmonary heart disease, previous history of thrombosis, mMRC score (Table 1), the last year times of acute exacerbations, GOLD classification based on spirometry ( patients with FEV1/FVC < 70%; I: FEV1≥80% predicted; II: 50% ≤ FEV1< 80% predicted; III: 30% ≤ FEV1< 50% predicted; IV: FEV1<30% predicted). The judgement of clinical symptoms was based on the definition of diagnosis. The results of laboratory tests were provided by the laboratory of our hospital. Prolonged immobility ≥3 days means bedtime >50% during the day and cannot be fully self-care. Diabetes, hypertension, pulmonary heart disease, and pulmonary encephalopathy were counted by discharge diagnosis.
Grade
Projects
0
I'm breathless only when strenuous exercise;
1
I'm breathless when hurrying or walking up a hill;
2
I'm slower than my peers when walking because of shortness of breath, or I need to stop and rest;
3
I need to stop and rest after walking 100 meters or minutes on a flat ground;
4
I can't leave the home because of severe or when I get dressed or undressed;
Table 1: mMRC score. mMRC 0-1 grade is less symptom; mMRC = grade 2 is more symptom.
Statistical Analysis
All data were analyzed using SPSS 23.0. Normal distribution data were expressed as mean ± sd (Standard Deviation) while non-normal distribution data were expressed as median. The difference between AECOPD and AECOPD with PE was assessed with Student’s t-test, whereas differences between medians were evaluated with the Mann- Whitney test. The Pearson’s chi-squared (Χ²) test was applied for comparative analysis of categorical variables. The multivariate logistic regression analysis was performed for determining risk factors for PE in AECOPD patients; the Odds Ratios (OR) and 95% Confidence Intervals (95% CI) were calculated, and a P-value of <0.05 was regarded as significant.
Results
Comparing the clinical information of patients in AECOPD and AECOPD with PE, the in-hospital mortality rate of AECOPD with PE complication was 19.15% (9/47), which was significantly higher than AECOPD patients without PE (1.36%). Patients with PE were older (79.89±7.75 years, P=0.005), and the symptoms, including cough, dyspnea, chest pain, hemoptysis, syncope, and ECG performance were pointedly different among both groups (P < 0.05) (Table 2).
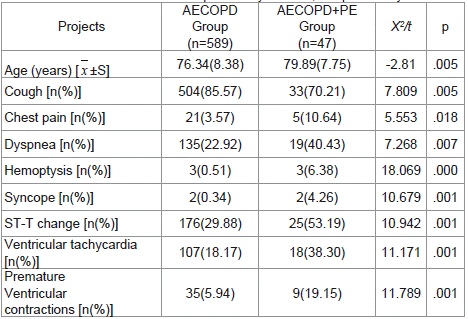
Table 2: Comparison of symptoms and electro cardiograms. AECOPD: acute
exacerbation of chronic obstructive pulmonary disease; PE: pulmonary embolism.
We also analyzed the results of laboratory tests in the two groups. Though the majority of laboratory indexes were not much different, the PH was lower while the PaCO2, D-dimer, and NT-proBNP levels were greater in patients with PE (P <0.05) (Table 3). Comparing signs, past history, and comorbidities between the two groups, we found that wet rales, pleural effusion, asymmetric lower extremity edema, history of stroke, pulmonary heart disease, pulmonary encephalopathy, and prolonged immobility ≥3 days were more common in AECOPD patients with PE complication (P<0.05). The days of hospitalization (14.36±1.12 days, P ≤0.001) and the disease course (16.21±1.61 years, P =0.021) were longer in the AECOPD patients with PE. No statistical differences were observed in coronary heart disease, hypertension, diabetes, malignant tumor, etc (P >0.05) (Table 4).
Projects
AECOPD Group
(n=660)AECOPD+PE Group
(n=47)t
P
PH
7.40(0.062)
7.35(0.13)
4.312
.000
PaCO2 (kPa)
6.34(0.08)
7.48(0.53)
-3.442
.001
PaO2((kPa)
11.24(0.16)
10.74(0.92)
0.812
.417
D-dimer (mg/l)
0.37(0.02)
1.14(0.19)
-10.008
.000
Fbg(pg/l)
4.74(0.13)
4.39(0.24)
0.764
.445
NT-proBNP(pf/ml)
1248.67(109.34)
2988.77(597.28)
-4.125
.000
WBC(×109/l)
10.89(1.36)
9.09(0.66)
0.371
.711
CRP(mg/l)
68.58(6.41)
64.73(9.24)
0.1698
.866
Table 3: Comparison of laboratory test data between two groups [ x ±S]. PH: potential of hydrogen; PCO2: partial pressure of carbon dioxide; PO2: arterial partial pressure of oxygen; Fbg: Fibrinogen; NT-proBNP: N-terminal B-type natriuretic peptide; WBC: leukocyte; CRP: C-Reactive protein.
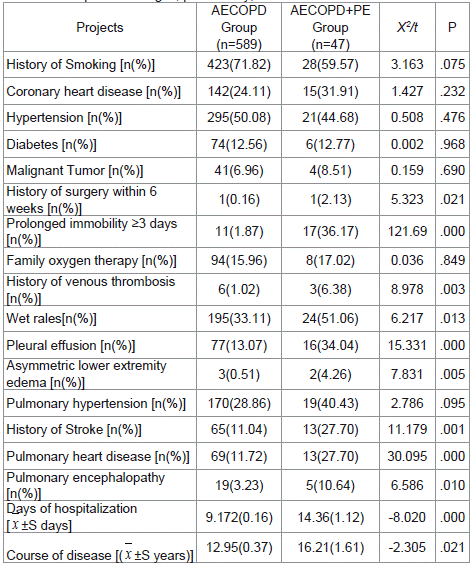
Table 2: Comparison of signs, past history, and comorbidities.
After analyzing the GOLD classification, mMRC score, and last year’s times of acute exacerbations in the two groups, we interestingly found that in the GOLD grade, patients with grades III and IV were not more inclined to PE. There was no difference in GOLD grade (P =0.555) between the two groups. However, the mMRC score and last year’s times of acute exacerbations showed statistical significance between both groups (P <0.05) (Table 5).
Projects
Cases (n)
AECOPD Group
(n=589)AECOPD+PE Group
(n=47)X2/t
P
GOLD grade
2.085
.555
I
3
3(0.51)
0(0)
II
123
113(19.19)
10(21.28)
III
340
319(54.16)
21(44.68)
IV
170
154(26.14)
16(34.04)
mMRC score (grade)
17.038
.002
0
43
43(7.30)
0(0)
1
108
103(17.49)
5(10.64)
2
210
196(33.28)
14(29.79)
3
238
218(37.01)
20(42.55)
4
37
29(4.92)
8(17.02)
Annual acute exacerbation times
37.774
.000
0
190
184(31.24)
6(12.77)
1
233
224(38.03)
9(19.15)
2
159
140(23.77)
19(40.43)
3
38
30(5.09)
8(17.02)
4
10
7(1.19)
3(6.38)
5
6
4(0.68)
7(4.26)
Table 5: GOLD Grading and mMRC Score [n(%)].
Further logistic regression analysis of the above statistically significant indicators showed that cough (OR=0.338, 95% CI: 0.106~1.074, P=0.036), chest pain (OR=8.171, 95% CI: 1.684~39.652, P=0.009), syncope (OR=75.262, 95% CI: 1.509~3753.780, P =0.030), premature ventricularcontractions (OR=6.683, 95% CI: 1.678~26.626, P =0.007), prolonged immobility ≥ 3 days (OR =10.679, 95% CI: 1.866~61.130, P=0.008), history of stroke (OR=4.247, 95% CI: 1.458~12.370, P =0.008), pulmonary heart disease (OR = 0.023, 95% CI: 0.002~0.241, P = 0.002), pulmonary encephalopathy (OR=4.467, 95% CI: 1.028~9.422, P=0.046), plasma D-dimer level (OR =3.193, 95% CI: 1.784~5.714, P ≤0.001), and the last year times of acute exacerbations (OR =1.808, 95% CI: 1.118~2.926, P ≤0.001) were independent risk factors for AECOPD with PE complication (Table 6).
Projects
Regression coefficient
Standard error
Wals values
P
OR
95% CI EXP (B)
Lower limit
Upper ceiling
D-dimer (mg/L)
1.161
0.297
15.280
.000
3.193
1.784
5.714
Days of hospitalization (days)
0.194
0.054
12.799
.000
1.214
1.092
1.350
Cough
-1.085
0.590
3.382
.036
0.338
0.106
1.074
Chest pain
2.101
0.806
6.794
.009
8.171
1.684
39.652
syncope
4.321
1.995
4.693
.030
75.262
1.509
3753.780
premature ventricular contractions
1.900
0.705
7.255
.007
6.683
1.678
26.626
Prolonged immobility =3 days
2.368
0.890
7.078
.008
10.679
1.866
61.130
History of Stroke
1.446
0.545
7.030
.008
4.247
1.458
12.370
Pulmonary encephalopathy
1.497
0.750
3.985
.046
4.467
1.028
19.422
Pulmonary heart disease
-3.765
1.194
9.937
.002
0.023
0.002
0.241
Annual acute exacerbation times
0.592
0.245
5.824
.016
1.808
1.118
2.926
Constant
-12.081
29.705
0.165
.684
0.000
Table 6: Logistic regression analysis. In regression analysis, AECOPD with PE was assigned to 1, and AECOPD alone was assigned to 0, it was a dependent variable. Age, pulmonary encephalopathy, prolonged immobility ≥3 days, history of stroke, pulmonary heart disease, history of stroke, cough, chest pain, syncope, premature contractions as an independent variable, in the presence of assignment 1, no assignment.
Discussion
COPD patients always experience chronic hypoxia, which leads to extensive pulmonary vasoconstriction, pulmonary hypertension, vascular intimal thickening, red blood cell compensatory proliferation, and eventually leads to increased blood viscosity, high blood coagulation, and slow blood flow [8]. Moreover, when the patients are in the acute exacerbation period, the inflammation and hypoxia will be further aggravated, and the patient’s active endurance will be further reduced, leading to an increased risk of pulmonary thrombo embolism in such hospitalized patients.
A total of 636 patients were enrolled in this survey, most patients admitted to the hospital for AECOPD were GOLD grade III-IV (510/636). The occurrence of PE in AECOPD patients was 7.40% (47/636), which was lower than the findings of scholars who reported an incidence of 10.3% (54/522) [9]. The major reason for this difference may be the differences in the research objects, and because we did not receive PE screening for all patients. In our study, 8 patients without pulmonary embolism died during hospitalization, the mortality rate was 1.36% (8/589), 9 patients with pulmonary embolism died during hospitalization, the mortality rate was 19.15% (9/47), which was significantly higher than that of patients without pulmonary embolism (P ≤0.001).
Previous studies on risk factors for AECOPD complicated with pulmonary embolism are limited. Some scholars confirmed that D-dimer levels, smoking history, prolonged bed rest, diabetes mellitus symmetrical lower extremity edema, pH, NT-proBNP levels, aortic pulmonary/ascending aorta >1, aorta/ascending aorta> 1, unequal thickness of lower limbs (≥1cm), and PaCO2 level (<35mmHg) in arterial blood gas were independent risk factors of AECOPD with Deep Vein Thrombosis (DVT) [9-12]. In our study, we found that chest pain, syncope, premature ventricular contractions, prolonged immobility ≥3 days, stroke history, pulmonary heart disease, pulmonary encephalopathy, and plasma D-dimer levels were independent risk factors for AECOPD with PE.
Moreover, our findings differ from other studies in the fact that times of acute exacerbations in the last year and mMRC scores showed a great difference between the two groups (P <0.05). In addition, the more acute exacerbations times and the higher the mMRC score, the higher risk of PE in AECOPD patients. This has not been reported in previous studies. Therefore, when the times of acute exacerbation of patients in the last years are significantly increased or the mMRC score is higher, it always indicates that the patient’s condition has worsened and it is necessary to pay attention to whether the AECOPD patient has PE complications.
Pulmonary Embolism (PE) is a life-threatening clinical manifestation often with non-specific clinical symptoms and signs, which may be difficult to diagnose early. The current data show that the three-month mortality rate for PE patients with stable hemodynamics is between 6% to 11%, while for PE patients with unstable hemodynamics or shock the mortality is 30% or higher [13].
Undeniably, in different studies, because of the differences in race, sample size, study design, health care policy levels in different regions, and case inclusion, the present results show significant differences in the incidence of PE in AECOPD patients. Consequently, the interpretation of the results of this study is limited, numerous factors may explain the discrepancy: (1) the sample size is small; (2) single center clinical data (the patients we admitted are all moderate to severe acute exacerbation, chronic hypoxia and hypoactivity, and all patients with a high risk of thrombus). Our small sample size limits universality, and differences in patient demographics may also affect the results of our study. In future studies, larger and more diverse patients will be needed to verify the reliability of our conclusions.
Conclusions
PE is a frequent event in AECOPD patients. When AECOPD patients show symptoms such as chest pain, syncope, premature contractions, prolonged immobility ≥ for 3 days, a history of stroke, pulmonary heart disease, pulmonary encephalopathy, increased times of acute exacerbation in the last year, high mMRC score and elevated plasma D-dimer levels, we should be alert for complicated PE. It is recommended to use prophylactic anticoagulation therapy to improve the prognosis of patients and reduce the mortality rate of AECOPD patients with high risk factors.
Author Contribution
Ling Peng and Chao Zhou conceived and coordinated the study, designed, performed and analyzed the experiments, and wrote the paper. An Wang, Fei Xue, and Ya-Fang Miao carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Disclosure of Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by Pudong new area Science, Technology and Economy Commission Shanghai (No: PKJ2020-Y29).
References
- Chinese Expert Consensus Compilation Group for Acute Exacerbations of Chronic Obstructive Pulmonary Diseases. Chinese Expert Consensus for Acute Exacerbations of Chronic Obstructive Pulmonary Diseases. International Journal of Respiration, 2019; 39: 1281-1296.
- Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008; 358: 1037-52.
- Chen W, Lin C, Lin C, Chang Y, Sung F, Kao C, et al. Pulmonary Embolism in Chronic Obstructive Pulmonary Disease: A Population-Based Cohort Study. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2014; 11: 438- 443.
- Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135: 786-793.
- Mispelaere D, Glerant JC, Audebert M, et al. Pulmonary embolism and sibilant types of chronic obstructive pulmonary disease decompensations. Rev Mal Respir. 2002; 19: 415-23.
- Cao Y, Dong L, Cao J. Pulmonary Embolism in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Chinese Medical Journal. 2018; 131: 1732-1737.
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2020.
- Li H, Zeng Z, Cheng J, Hu G, Li Y, Wei L, et al. Prognostic Role of NT-proBNP for in-Hospital and 1-Year Mortality in Patients with Acute Exacerbations of COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2020; 2020: 57-67.
- Li YX, Zheng ZG, Liu N, et al. Analysis of risk factors for acute exacerbation of chronic obstructive pulmonary disease with pulmonary embolism. Chinese Journal of Tuberculosis and Respiratory, 2016; 39: 298-303.
- Zhu XM, Luo J, Li MM, et al. Meta analysis of risk factors for venous thromboembolism in patients with chronic obstructive pulmonary disease. Chin J Nurs. 2019; 54 5.
- Lin SF, Guo HY, Gan ZY, et al. Analysis of risk factors for acute exacerbation of chronic obstructive pulmonary disease and pulmonary embolism. Chinese Community Physicians. 2019; 35: 5.
- Zhang ZF. Clinical characteristics of acute exacerbation of chronic obstructive pulmonary disease with pulmonary embolism. Journal of Dalian Medical University. 2019.
- Qin J, Liang H, Shi D, Dai J, Xu Z, Chen D, et al. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. Journal of Thrombosis and Thrombolysis. 2014; 39: 215-221.