
Special Article - Stereotactic Body Radiotherapy
Austin J Radiat Oncol & Cancer. 2015;1(2): 1008.
Radiosurgery for Patients with Oligometastasis to Retroperitoneal Lymph Nodes
Barrios CJ¹, LaCouture T², Patel A², Goldsmith B², Asbell S², Xu Q², Warshal D³ and Kubicek GJ²*
¹Cooper Medical School of Rowan University, Camden, New Jersey
²Department of Radiation Oncology, Cooper University Hospital, New Jersey
³Department of Gynecology-Oncology, Cooper University Hospital, New Jersey
*Corresponding author: Kubicek GJ, Department of Radiation Oncology, Cooper University Hospital, Camden, NJ, United States of America
Received: April 04, 2015; Accepted: April 29, 2015; Published: April 29, 2015
Abstract
Purpose: We report both the control and the toxicity of Stereotactic Body Radiotherapy (SBRT) therapy in a series of oligometastatic cancer patients with Retroperitoneal lymph Node (RPLN) involvement.
Methods: This is a retrospective review of a prospective database. Eligible patients for review received SBRT to metastatic RPLN. 26 patients with RPLN failure (median age: 61, 3 males) were treated with SBRT. Treatment was to an isodose line (median: 65%). Median dose was 27.5 Gy (range: 15-54 Gy) and median fractions were 5 (range: 3-5).
Results: 26 patients were analyzed with a median follow-up of 13 months and 22 months for surviving patients. 16 patients (61.5%) had distant cancer recurrence after SBRT, while 5 patients (19.2%) had a local recurrence at the site of SBRT. Six patients (23%) had grade 2 toxicities, including nausea/vomiting, constipation, and abdominal pain. There were no grade 3 or higher toxicities and no late toxicities. Overall survival was improved in patients with distant control (median survival for patients with distant failure was 430 days versus 845 days for patients without distant relapse, P < 0.047). Higher Biological Equivalent Dose (BED) predicted for improved local control (P < 0.027), we did not find any local failures with a BED-10 greater than 50 Gy.
Conclusion: SBRT in the treatment of RPLN recurrences is safe and effective. We recommend treatment to a dose greater than BED-10 50 Gy, our preferred dose regimen is 35 Gy in 5 fractions. Improved distant control was associated with an improved OS in this series.
Keywords:
Radiosurgery; Cyberknife; OligometastaticAbbreviations
BED: Biological Effective Dose; GTV: Gross Tumor Volume; Gy: Gray; KPS: Karnofsky Performance Status; OS: Overall Survival; PTV: Planned Tumor Volume; RPLN: Retroperitoneal Lymph Node; SBRT: Stereotactic Body Radiotherapy
Introduction
Aggressive treatment for patients with limited metastatic disease (oligometastatic) is an ongoing area of research. Multiple studies have found that a subset of patients with oligometastatic disease will remain disease free on long-term follow-up. In a majority of these studies, evidence has shown that local therapy may even be curable for a subset of patients with local metastasis [1,2]. For example, D’Angelica et al. [1] found that some patients who underwent surgical resection for liver metastasis from colorectal cancer could remain disease free 5 years after local treatment.
One of the common methods for treating oligometastatic sites is Stereotactic Body Radiotherapy (SBRT), which entails high dose radiation with sub-centimeter precision. SBRT has been shown to be effective in the treatment of oligometastatic disease in several body sites including lung [3,4], liver [5,6], adrenal gland [7] and Retroperitoneal Lymph Nodes (RPLN) [8,9]. While not the most common site for oligometastatic disease, RPLN can be a site of distant failure, especially for cancers in the pelvis.
The effectiveness and use of SBRT in patients with metastasis to RPLN has not widely been reported in the literature. Bignardi et al. [8] reported on 19 patients with unresectable RPLN treated with SBRT (45 Gy in 6 fractions) and found local control in 17 patients, 11 patients with progression at distant sites, and limited toxicity. Jereczek-Fossa et al. [9] reported on 69 patients with a single RPLN treated with SBRT (median 24Gy/3 fractions) and found 75.3% infield control. 49.3% of patients had no toxicity at all and 2 patients had grade 3 acute toxicity (genitourinary) and one patient with late grade 4 toxicity (hemorrhagic duodenitis and duodenal substenosis).
The purpose of this study is to explore both the effectiveness and toxicity of SBRT in patients with oligometastasis to RPLN.
Methods and Materials
This is a retrospective review of a prospective database (IRB EX-1094). All patients signed consent to allow data collection on this database. Patient treatments and background information were prospectively collected while other information (patterns of failure, late toxicity, survival) was retrospectively gathered. Patients eligible for this review had SBRT treatment to a RPLN at our institution between 1/1/2009 and 9/1/2013. The database consisted of a total of 867 patients. Of these, 29 patients met eligibility criteria (treatment to RPLN), 3 patients were excluded for missing data leaving 26 patients eligible for this analysis. Median age was 61 years, 10 patients had gynecologic primaries, 3 patients were male, and the majority of patients had previous treatment including 15 with previous chemotherapy, 13 with previous radiation and 20 with previous abdominal surgery. Characteristics of analyzed patients are in (Table 1).
Gender
Number (%)
Male
3 (11.5%)
Female
23 (88.5%)
Previous radiation
Yes
13 (50%)
No
13 (50%)
Previous chemotherapy
Yes
15 (57.7%)
No
11 (42.3%)
Previous pelvic- abdominal surgery
Yes
20 (76.9%)
No
6 (23.1%)
Tumor primary
GU
2 (7.7%)
GI
6 (23.1%)
Breast
1 (3.8%)
Lung
2 (7.7%)
Sarcoma
1 (3.8%)
Gyn
10 (38.5)
Other
4 (15.4%)
Other sites of metastatic disease at time of treatment
Yes
13 (50%)
No
13 (50%)
KPS at time of treatment
70
3 (11.5%)
80-100
14 (53.9%)
KPS unknown
9 (34.6%)
Age
Median
61
Range
32-84
Table 1: Patient characteristics.
Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Survival and failure were measured from the end of SBRT. Failure was defined as distant if the new site of metastatic disease occurred outside of the treated RPLN and local if there was progression of the treated RPLN. All treatments were performed on the CyberKnife SBRT system (Accuray, Sunnyvale, CA).All patients had CT simulation prior to SBRT. Gross Tumor Volume (GTV) was defined as actual gross disease as defined on CT scan and other imaging when available (for example, PET scan). A small margin was applied to the GTV to get a Planning Treatment Volume (PTV) and was typically 5mm in all directions, the PTV margin was physician dependent and some patients had smaller PTV margins. Spine tracking was used for treatment tracking (fiducial markers were not used). Most treatments were every other day. Dose constraints were placed on small bowel, spine; spine dose was limited to 28 Gy in 5 fractions and 18 Gy in 3 fractions, small bowel dose limits varied depending on treating physician. The median number of fractions was 5 (range: 3-5) and the median dose was 27.5 Gy (range: 15-54 Gy). Treatment was to an isodose line (median: 65range: 54- 94). We calculated the Biological Effective Dose (BED) using the formula:
Using α/β of 10 for acute responding tissue (cancer equivalent), D is total dose, and d is dose per fraction. Using this formula, the median BED-10 was 42 (range: 28-112). Treatment characteristics are in (Table 2).
Number of fractions
Number (%)
3
4 (15.4%)
4
1 (3.8%)
5
21 (80.8%)
Median
5
Radiation Dose (Gy)
15-20
5 (19.2%)
21-25
7 (26.9%)
26-30
8 (30.9%)
31-40
3 (11.5%)
>40
3 (11.5%)
Median
27.5 Gy
Isodose line
Median
65
Range
54-94
GTV volume (cc)
Median
30.1
Range
5.4-210.0
PTV volume (cc)
Median
65.4
Range
5.5-267.8
Small bowel dose (Gy)
Median
4.94
Maximum Dose (range)
Radiation BED (a/b 10)
15-30
6 (24%)
31-45
8 (32%)
45-60
- (20%)
>60
6 (24%)
Median
42
Table 2: Treatment characteristics.
Statistics were performed using SPSS version 22 (IBM San Jose, CA). For survival Kaplan-Meier was used with log-rank test to compare between variables. Independent T test was used to distinguish differences between means of continuous variables (such as radiation dose) and chi-squared test utilized to test significance between categorical variables.
Results
Median follow-up for all patients was 13 months (range: 2.7 to 40.1 months) with a median follow-up of 22 months for surviving patients. At last follow-up 11 patients were alive and 6 patients were alive and free of disease. Median Overall Survival (OS) for all patients was 845 days (Figure 1). We looked at multiple pre-treatment factors that could be associated with OS including age, Karnofsky Performance Status (KPS), primary type, previous chemotherapy, previous surgery, previous radiation, other areas of disease at time of treatment, time from initial diagnosis. None of these pre-treatment factors were significant for differences in OS. Post-treatment factors including radiation dose, BED dose, small bowel dose, local failure, distant failure, treatment volume, post-treatment chemotherapy were examined. Significant factors for worse OS included distant failure (p< 0.047) (Figure 2), none of the other factors were significantly associated with changes in OS although post-treatment chemotherapy had a trend towards improved OS with a median OS of 796 days for patients receiving post SBRT chemotherapy and 501 days for patients that did not receive post SBRT chemotherapy (p = 0.125).
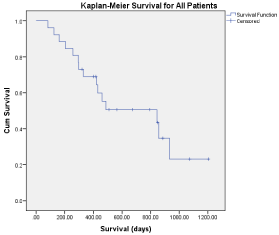
Figure 1: Kaplan-Meier survival (all patients).
Sixteen patients (61.5%) had distant progression of their cancer following SBRT therapy. Multiple variables were examined in relation to distant failure. There was a worse OS in patients with distant progression (Figure 2), median survival for patients with distant failure was 430 days versus 845 days for patients without distant relapse (P < 0.047).The only other statistically significant factor was the use of chemotherapy post SBRT. The use of chemotherapy following SBRT was shown to reduce the rate of distant progression (p < 0.008).
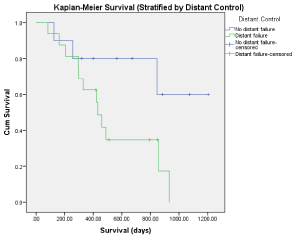
Figure 2: Kaplan-Meier survival (stratified by distant control).
Five patients (19.2%) had local failure as defined by progression of treated RPLN. There was no association between local failure and OS. Patients with local failure had a median radiation dose of 27.5 Gy (range: 25-30 Gy) while patients without failure had a median dose of 25 Gy (range: 15-54 Gy). The BED-10 dose for patients with failure was 37.5 (range: 28-48) and for patients without local failure 45 (range: 19.5-126). We did not have any local failures with BED-10 greater than 50. Using an independent T test, BED-10 was statistically significant (p< 0.027) predictor for local failure with higher BED-10 associated with decreased local failure rates.
The median GTV volume was 30.1 cc (range 5.4-210.0). Median PTV volume was 65.4cc (range: 5.5-267.8cc). There was a trend for improved local control for lower PTV and GTV volumes. Median PTV volume for patients with local failure was 163.0 cc (range 34.0 to 267.8 cc) versus 80.3 cc (range 5.5 to 239.0) for patients without local failure (P = 0.061). Median GTV volume for patients with local failure was 112.7 cc (range 12.0 to 195.4 cc) and 52.3 cc (range 5.4 to 171 cc) for patients without local failure (p = 0.08). There was no trend or association between distant failure and PTV or GTV.
Using the CTCAE, only 6 patients had acute toxicity. All 6 had acute grade 2 toxicities that included nausea, vomiting, constipation, and abdominal pain. There was no correlation between radiation dose, bowel median or maximum dose, BED dose, and toxicity. In the remaining 20 (76%) patients, no toxicities were reported. There was no late toxicity. The organs at risk for treatment included small bowel and spinal cord. The spinal cord limit was 28 Gy in five fractions and 18 Gy in 3 fractions, none of the patients had a spinal cord dose exceeding this limit. Median small bowel dose was 4.94 Gy(range: 3.7 to 11.3) and the median maximum small bowel dose was 23.8 Gy (range 10.4 to 35.6 Gy).There was no relation between treatment volume (GTV and PTV), radiation dose, BED dose, small bowel dose, and acute toxicity.
Discussion
The treatment of oligometastatic disease is an area of ongoing research. There are multiple available reports (retrospective and single arm prospective) that show a subset of oligometastatic patients that do very well with aggressive treatment to all sites of disease. The biggest question, which is whether any patients benefit from aggressive therapy to oligometastatic disease, has never been addressed in a randomized trial, but there are some comparative effectiveness studies that show a benefit to aggressive therapy for oligometastatic disease. A report from MD Anderson [10] looked at propensity matched patients who had comprehensive local therapy to oligometastatic disease versus similar patients who did not have aggressive local therapy and found improved overall and progressionfree survival for patients with aggressive local therapy.
Until level one evidence for aggressive local treatment for oligometastatic disease is available it becomes important to use the evidence available in order to determine the best methods for oligometastatic treatment. The purpose of our report was to analyze treatment of RPLN oligometastatic disease. We found the treatment to be very well tolerated with minimal toxicity. Acute toxicity was mainly gastrointestinal in nature (grade 2 in 6 patients), and we did not have any late toxicity. Our control rates were very good with an overall control of 80.7% and no local failures for patients with radiation dose BED-10 greater than 50.Most of the treatment failures were distant and, perhaps not surprisingly, distant failure was correlated with a worse overall survival (Figure 2). It is also not surprising that use of chemotherapy post SBRT resulted in reduced distant recurrence, as more chemotherapy and targeted therapy agents become available more patients are likely to be candidates for some type of systemic therapy, likely making local control more important.
Our results agree with the limited data that has been published on SBRT for RPLN [8,9]. Bignardi et al. [8] described SBRT treatment of 19 patients with RPLN treated to 45 Gy in 6 fractions (BED-1078 Gy) and found 2 local failures in 19 patients and low rates of toxicity. Jereczek-Fossa et al. [9] also reported low toxicity rates and high control rates in 69 patients treated with SBRT (median dose 24 Gy in 3 fractions, BED-10 43.2) for RPLN. This group found 49.3% of patients to have no toxicity and 1 year local control of 81.6%. While Jerezcek-Fossa et al. did not find any relation between dose and control rate they did find that GTV volume was inversely correlated with local progression. While we did not find a statistically significant association between volume and local control but did note a strong trend in that direction, it is likely that with larger numbers this would have reached significance. SBRT results in treating RPLN show similar control rates to conventional radiation but with much less acute toxicity [11,12].
Obvious limitations of this study include the small number of patients and heterogeneous nature of the treatments. Also, some of the information was retrospectively obtained (such as toxicity) which is always subject to error. We feel that this report can help further improve treatments for oligometastatic patients with RPLN. Patients considering this treatment option can be told that treatment has minimal toxicity but should also be told that they have a high rate of distant failure. We recommend a radiation dose with greater than 50 BED-10, such as 35 Gy in 5 fractions.
References
- D’Angelica M, Brennan MF, Fortner JG, Cohen AM, Blumgart LH, Fong Y. Ninety-six five-year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg. 1997; 185: 554-559.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. Oct. 2007; 25: 4575-4580.
- Hof H, Hoess A, Oetzel D, Debus J, Herfarth K. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther Onkol. 2007; 183: 673-678.
- Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a non-invasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys. 2004; 60: 186-196.
- Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007; 67: 793-798.
- Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, et al. Stereotactic radiotherapy for primary liver cancer and hepatic metastases. Acta Oncol. 2006; 45: 838-847.
- Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006; 45: 823-830.
- Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys. 2011; 81: 831-838.
- Jereczek-Fossa A, Piperno G, Ronchi, S, Catalano G, Fodor C, Cambria R, et al. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrence cancer. Am J Clin Oncol. 2014; 37: 227-233.
- Sheu T, Heymach JV, Swisher SG, Rao G, Weinberg JS, Mehran R, et al. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int J Radiat Oncol Biol Phys. 2014; 15; 90: 850-857.
- Yeo SG, Kim DY, Kim TH, Jung KH, Hong YS, Kim SY, et al. Curative chemoradiotherapy for isolated retroperitoneal lymph node recurrence of colorectal cancer. Radiother Oncol. 2010; 97: 307-311.
- Lee J, Chang JS, Shin SJ, Lim JS, Keum KC, Kim NK, et al. Incorporation of radiotherapy in the multidisciplinary treatment of isolated retroperitoneal lymph node recurrence from colorectal cancer. Ann Surg Oncol. 2015.