
Special Article - Stereotactic Body Radiotherapy
Austin J Radiat Oncol & Cancer. 2015;1(2): 1008.
Linear Accelerator Stereotactic Radiosurgery in Intraocular Malignant Melanoma
Alena Furdová¹*, Miron Sramka², Martin Chorvath² and Gabriel Kralik³
¹Department of Ophthalmology, Comenius University, Slovak Republic
²Department of Stereotactic Radiosurgery, St. Elisabeth Cancer Inst. and St. Elisabeth University College of Health and Social Work, Slovak Republic
³Faculty of Medicine of the Slovak Medical University, Slovak Republic
*Corresponding author: Alena Furdová, Department of Ophthalmology, Medical School, Comenius University Pažítkova 4, 821 03 Bratislava, Slovak Republic
Received: May 05, 2015; Accepted: May 12, 2015; Published: May 13, 2015
Abstract
Objective: Linear accelerator based stereotactic radiosurgery of intraocular malignant melanoma is a method to treat uveal melanoma (ciliary body and choroid).
Material and Methods: Retrospective clinic-based study of patients with posterior uveal melanoma in stage T1 - T3 who underwent stereotactic radiosurgery at linear accelerator in period 2001- 2013.
Results: In group of 123 patients with posterior uveal melanoma treated with one day session stereotactic radiosurgery the median tumor volume was 0.6 cm3 (0.2 - 1.0 cm3). Patient age ranged from 25 to 82 years with a median of 55 years. The therapeutic dose applied to melanoma was 35.0 Gy, median maximal dose applied was 49.0 Gy (37.0 - 54.0 Gy). In the group of small tumors the volume regression was verified in 6 months and 12 months interval after the therapy by ultrasound and MRI (there was no presence of increase of the elevation). In 24 months interval the tumor regression was present in 25 cases from 35 cases (71.4 %). Tumor local control was successful in 95 % of patients in 2 years interval after stereotactic radiosurgery and in 85 % of patients in 5 years interval after stereotactic radiosurgery. Secondary enucleation due secondary glaucoma was necessary in 14 patients (11.4 %) in 3 to 5 year interval after irradiation.
Conclusion: One step LINAC based stereotactic radiosurgery with a single dose 35.0 Gy is treatment option to treat T1 to T3 stage intraocular melanoma.
Keywords: Intraocular tumors; Uveal melanoma; Linear accelerator radiosurgery
Introduction
Uveal melanoma is relatively rare type of cancer, but the most common and most aggressive type of intraocular tumor in adults. The incidence of intraocular tumors varies from 0.2 to 1.0. According to the Slovak National Cancer Registry the incidence in Slovakia is 0.2 to 0.6 / 100 000 inhabitants. The recorded data from Slovak regions correspond with the data reported from other countries and regions of Europe [1].
Over 50 % of patients with uveal melanoma die within 15 years after the therapy – either radical surgery (enucleation), or other therapeutical methods [2]. Age and volume (size) of the tumor have been shown to be prognostic indicators following therapy for posterior uveal melanoma. Modern diagnostic tools, ophthalmological examination, computed tomography and magnetic resonance have led to significant advances in the ability to diagnose primary uveal melanoma. Over the past three decades diagnostic methods have improved and radiotherapy (external beam, charged particle or brachytherapy) has become the preferred treatment for most patients with uveal melanoma. The desire to improve survival and preserve vision in patients with uveal melanoma has stimulated the development of alternative therapies. Different radiation modalities are currently in use in treatment of posterior uveal melanoma. One of the methods of “conservative” approach is the Stereotactic Radiosurgery (SRS) by linear accelerator.
Stereotactic radiation therapy and gamma-knife radiosurgery also provide good local control, with survival rates comparable with other treatments.
SRS of extracerebral lesions like uveal melanoma has been invented in the last two decades and is an alternative treatment for middle and large posterior choroidal melanoma. With plaque radiotherapy, eye salvage is achieved, and, particularly for cases in which the tumor is located away from the optic disc or macula, useful vision can be retained after treatment. The single irradiation of the intraocular tumor by linear accelerator therapy itself is a new approach – it has been shown to achieve ultrasonic tumor regression in a similar fashion to brachytherapy.
In this study we assess the treatment of posterior uveal melanoma by one-day session of LINAC based stereotactic radiosurgery.
Methods
A retrospective analysis was undertaken for patients with posterior uveal melanoma (tumor arising from ciliary body or choroid) in stage T1 to T3 who underwent stereotactic radiosurgery at C LINAC in period 2001- 2013. Patients were not randomized either to radical (enucleation) or to “conservative” procedure, but the treatment was determined exclusively on a case-by-case basis. Tumor stage, volume, maximum elevation, localization presence of secondary retinal detachment, general status, age, gender, the functional tests (visual acuity, perimeter, ultrasound) were taken into consideration. The patient was actively involved in the decision on the therapeutic procedure after explaining possible postoperative complications.
Before stereotactic irradiation immobilization of the affected eye was achieved by mechanical fixation to the stereotactic Leibinger frame. Sutures were placed under 4 direct extraocular muscles through conjunctiva and through the lids. The stereotactic frame was fixed to the head and the sutures were tied to the stereotactic frame (Figure 1). The patient underwent CT and MRI examination with the fixed eye to the frame. Stereotactic radiosurgery was perfomed by oned – day session on linear accelerator Model LINAC C 600 C/D Varian with 6 MeV X. The stereotactic treatment planning after fusion of CT and MRI was optimized according to the critical structures - lens, optic nerve, also lens and optic nerve at the contralateral side, chiasm (Figure 2a and 2b). The best stereotactic radiosurgical planning scheme was applied for therapy at linear accelerator. Tumor volume calculation was based on the ROI (region of interest) of the tumor and 3D reconstruction was done. The planned therapeutic dose into the tumor mass was 35.0 Gy by 99 % of DVH (dose volume histogram).
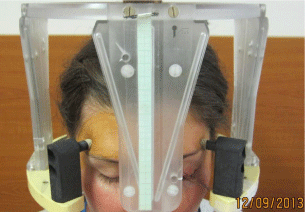
Figure 1: Patient with stereotactic frame, the left eye is immobilized through
stitches to the frame.
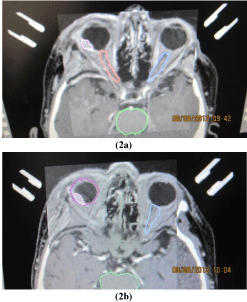
Figure 2: Small choroidal melanoma on the posterior pole of the eye – tumor
and critical structures (optic nerves).
The stereotactic treatment planning after fusion of CT and MRI was optimized according to the critical structures (lens, optic nerve, and also lens and optic nerve at the contralateral side, chiasm). The best plan was applied for therapy at C LINAC accelerator (Figure 3,4a,4b,4c and 5). In the afternoon the patient underwent irradiation at linear accelerator. Sutures and frame were removed. Next day the patient underwent the examination by an ophthalmologist - the slit lamp examination, ophthalmoscopy, intraocular pressure measuring and was released for home treatment with local therapy (eye drops - antibiotics, corticosteroids, lubricant).
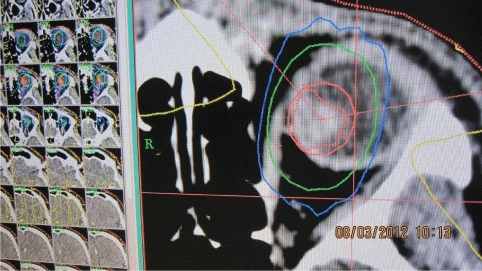
Figure 3: Stereotactic planning scheme for the intermediate uveal melanoma.
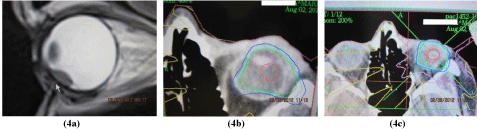
Figure 4: Small ciliary body melanoma: a) CT scan before treatment, b)
intraocular critical structures – lens, c) stereotactic planning scheme.
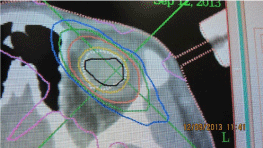
Figure 5: Stereotactic planning scheme for large uveal melanoma.
We used PTV (planning treatment volume) 95% isodose planning. The planned therapeutic dose in SRS was 35.0 Gy, TDmin. dose to the margin of the lesion varied from 35.0 to 38.0 Gy, TDmax 37.0 - 50.0 Gy. The doses to the critical structures were below 8.0 Gy for the optic nerve and the optic disc and 10.0 Gy to the anterior segment of the eye. Patients with melanocytoma or patients with juxtapapillary melanomas were excluded from the study.
The record for each patient included the age at treatment, tumor size, tumor volume, the maximum height of the tumor by B scan ultrasound, the presence and the extent of secondary retinal detachment, and the signs of extrascleral extension. Tumor volume was calculated in each SRS group patient directly by computer after CT and MRI examination as the step of SRS procedure and was involved to the stereotactic planning scheme.
Tumors were divided into 3 groups as follows: small – up to 5 mm of maximal elevation, middle – up to 8 mm, and large – over 8 mm. The elevation of the tumor was observed in 6 months interval by an ultrasound by one ophthalmologist. We compared tumor regression by measuring the maximum elevation by Bscan ultrasound in the group of patients with single irradiation in interval 3, 6, 12 and 24 months after the therapy, or, in individual cases, every month, but 2 years after stereotactic radiosurgery patients were asked for examination at least 2 times per year.
Patients were recommended regularly in six month interval to their oncologist to scrren metastasis (liver ultrasound, abdominal ultrasound, liver’s function test; once per year chest X-ray ). In individual cases and young patients were recommended to whole body PET/CT(positron emission tomography).
Results
In a group of 123 patients with posterior uveal melanoma (ciliary body, choroid) treated with one day session stereotactic radiosurgery, patient’s age ranged from 25 to 82 years with a median of 55 years. Median tumor volume at baseline was 0.6 cm3 (with a range from 0.2 to 1.0 cm3). Median of the maximal dose applied was 49.0 Gy (ranged from 37.0 to 52.0 Gy).
Tumor local control was successful in 95 % of patients in 3 years interval after stereotactic radiosurgery and in 80 % of patients in 5 years interval after stereotactic radiosurgery.
Tumor regression in patients by Bscan ultrasound findings
Patients operated for uveal melanoma with single irradiation by SRS between 2001–20013 were divided into 3 subgroups according to the maximum elevation before irradiation. The ultrasound findings were correlated to CT or MRI findings before irradiation on linear accelerator and then every time by the visit by an ophthalmologist. Tumors were divide into groups: small – 4 to 5 mm high = 35 cases (28,4 %), middle - up to 8 mm high = 52 cases (42,3 %), large – over 8 mm = 36 cases (29,3 %) (Figure 6).
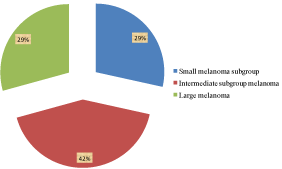
Figure 6: Patients with uveal melanoma indicated to stereotactic radiosurgery
at linear accelerator in 2001 – 2013 at the Dept. of Ophthalmology, Comenius
Univ. in Bratislava and Dept. of Stereotactic Radiosurgery, St. Elisabeth
Oncology Inst. in Bratislava.
Tumor regression after the treatment in 6 months interval and 12 months after the therapy showed, that in the group of small tumors in all of the patients there was no presence of increase of the elevation, but in 24 months interval there was sign of tumor regression in 25 cases from 35 cases (71,4 %). In the middle stage group in 12 months interval after the therapy there was no sign of tumor elevation regression, but in the 24 months interval after the therapy tumor regression more than 1mm of the maximum pre-treatment elevation was found in 36 cases from 52 cases (69,2 %). In the third subgroup of large tumors there was no sign of tumor regression according to ultrasound results in the 12 months or in the 24 months interval after the therapy.
Complications after stereotactic radiosurgery for uveal melanoma
Late complications were observed at the last follow-up examination like macular destruction because of scarring around the tumor, optic nerve atrophy, maculopathy, retinopathy, partial lens opacity, total cataract, vitreous hemorrhage, secondary glaucoma, thrombosis of the central retinal vein. Secondary enucleation in period 2001 – 2013 after stereotactic radiosurgery due to irradiation neuropathy and secondary glaucoma was necessary in 14 patients (11.4 %) in 3 to 5 year interval after irradiation. In all of the cases the tumors had pre-treatment maximum elevation 10 mm and more; the tumor volume was up to 0.7 mm3 (average 0.9 mm3). In 3 patients the tumor was arising from the ciliary body. There was no presence of optic nerve infiltration and extrascleral extension of the melanoma in all of the enucleated eye-globes.
Discussion
One-fraction LINAC radiotherapy/radiosurgery is arelatively unusual approach to treatment of choroidal melanoma. Image fusion of a contrast - enhanced magnetic resonance imaging and computed tomography is used for treatment planning co-ordinates. This treatment is used in a way of SRS with a single fraction administered with a precious spatial accuracy using a collimating system. No survival difference attributable to stereotactic irradiation or combined and surgical attitude - enucleation of uveal melanoma has been demonstrated in the retrospective study in Slovak Republic. Enucleation after SRS in 7 patients was in interval 6 to 24 months after SRS. A small difference is possible, but a clinically meaningful difference in mortality rates, whether from all causes or from metastatic melanoma, is unlikely [1].
High rates of local control can be achieved with 5-year control rates exceeding 95% in patients treated with charged particles. Proton beam radiotherapy with a 62 MeV cyclotron achieves high rates of local tumor control and ocular conservation, with visual outcome depending on tumor size and location [3].
Large, prospective, randomized trials were designed to compare mortality Figureures for medium-sized melanomas treated by brachytherapy or enucleation [4,2].
In the last three decades, the management of patients with uveal melanoma has changed towards globe sparing techniques. Alternatives to the radical enucleation vary from observation to transpupillary thermotherapy, block-excision, endoresection with pars plana vitrectomy, brachytherapy using a variety of radioisotopes, external beam radiotherapy, charged particles and stereotactic radiosurgery, or the methods can be combined. SRS has recently been proposed as an alternative treatment for posterior uveal melanoma.
The therapy for each patient should be chosen in accordance with the general status of the patient and with the local findings, stage and character of the tumor.
Stereotactic photon beam irradiation has been under clinical investigation for the treatment of uveal melanoma for over 15 years. Single-fraction Stereotactic Radiosurgery (SRS) is usually done with a gamma knife as well as more recently with a cyberknife. The therapeutic single dose has been reduced to as low as 35.0 Gy over the past few years without reduction in tumor control. Doses of 40.0 Gy delivered at the 50% isodose result in good local tumor control and acceptable toxicity. Since radiobiological studies indicate a possible advantage of hypofractionated treatment over a single very large fraction to sterilize uveal melanoma cell lines, fractionated Stereotactic Radiotherapy (SRT) has gained additional interest. Besides increased tumor control, toxicity should theoretically be reduced by fractionation. Linear Accelerators (LINAC) have the advantage of a feasible fractionation. Most LINAC studies employ a hypofractionated scheme of 4-5 fractions and total doses between 50.0 and 70.0 Gy. The efficacy of SRT for uveal melanoma has been proven in different studies with local tumor control rates reported over 90%, 5 and 10 years after treatment.
There has been performed no multicentre trial to assess dosimetry, safety and efficacy of SRS, or to evaluate outcomes of gamma knife radiosurgery for melanoma yet, but data from several reported case series suggest that SRS can have similar local tumor control rate, metastasis rate, mortality rate and complications rate when compared to brachytherapy. Recent studies have suggested that gamma knife radiosurgery and SRS may be an appropriate alternative for treating uveal melanoma in those patients, in whom lesions are ineligible for conventional brachytherapy. The findings in the series suggest a role of SRS in the treatment of selected cases of uveal melanoma.
Radiogenic side effects after SRT are reported similarly to other forms of radiotherapy, with cataract development, radiation retinopathy, opticopathy and neovascular glaucoma being responsible for the majority of secondary visual acuity losses and secondary enucleations. Overall, Stereotactic Photon Beam Radiotherapies (SRS and SRT) are considered effective treatment modalities for uveal melanoma, with promising late tumor control and toxicity rates.SRS is a relatively new method, so there is a need for multi-center trial to compare the outcomes following stereotactic radiosurgery with other methods. However, until now, no study has been performed in this topic. Studies comparing survival rates following enucleation versus newer treatment modalities, including SRS, suggested similar rates for comparable lesions and because reported local tumor control rate following SRS appear comparable, we offer SRS to patients who would otherwise require enucleation [1,4-6].
Stereotactic photon therapy of uveal melanoma, based on CT and MRI images, is a safe and precise treatment option. Local control was found to be excellent. In the study of [7] due to unfavorable tumor size and location in the vicinity of critical structures, e.g. optic nerve and macula, visual reduction was noticed in a high number of the patients. After an observation time of more than 6 months visual acuity could be evaluated in 79 patients. In the group of 77 patients 85.5 % presented with visual acuity of 0.1 or better prior to radiotherapy. LINAC based stereotactic irradiation for uveal melanoma is feasible and well tolerated and can be offered to patients with medium sized and unfavorably located uveal melanoma who are searching for an eye-preserving treatment. Because of selection criteria, the number of patients in the study with reduced visual acuity will probably increase in the future [8].
Local control over 95% appears in some studies: in the study of [8] local control is 98% after a median observation time 33 months follow up. The observation time is still too short to allow definitive conclusions, but their results are comparable with the 82 - 98 % local control rate reported by other groups after a median observation time of up to 15 years.
A retrospective study that irradiation of 30.0 Gy of more than 2 mm of the optic nerve head initiated an optic neuropathy [9].
There has been performed no multi-center trial to assess dosimetry, safety and efficacy of SRS, or to evaluate outcomes of gamma knife radiosurgery for melanoma yet, but data from several reported case series suggest that SRS can have similar local tumor control rate, metastasis rate, mortality rate and complications rate when compared to brachytherapy [10-12]. Recent studies have suggested that gamma knife radiosurgery and SRS may be an appropriate alternative for treating uveal melanoma in those patients, in whom lesions are ineligible for conventional brachytherapy [13- 15]. The findings in the series suggest a role of SRS in the treatment of selected cases of uveal melanoma.
The eye retention is one of the main goals of the conservative treatment, but in some cases enucleation can be indicated due to complications after therapy e.g. secondary neovascular glaucoma [16,17].
A multivariate data analysis by employing the supervised learning techniques, in particular the algorithm known as Regularized Least Squares (RLS) was used in study of [18]. Their study was the largest one in Italy and they demonstrated the excellent local tumor control, survival and eye retention rate after the proton beam irradiation therapy. According to their results future refinements in treatment planning, dosing and delivery could be necessary to determine visual results and complications after proton beam therapy in ocular melanoma.
The main issues with the single-session radiotherapy are the effects of distribution and hypofractionation of the dose. Tumor size and location, e.g. closer than 2 mm to the optic disc are the most important factors to assess clinical evaluation of visual acuity outcome. Identification of risk factors may reduce the rates of recurrence and lead to fewer complications, preservation of the eye, improved visual function and, potentially, better survival outcome The recurrence of optic neuropathy after stereotactic radiosurgery is a problem not only by intraocular tumors but also e.g. by perichiasmal tumors stereotactic irradiation. Although rare, optic neuropathy may follow radiosurgery to lesions near the visual pathways. Careful dose planning guided by MRI with restriction of the maximal dose to the visual pathways to less than 8.0 Gy will likely reduce the incidence of this complication [18,19].Stereotactic radiosurgery and fractionated stereotactic radiotherapy have emerged as promising, non-invasive treatments for uveal melanoma [20]. Although, historically, melanoma has been considered a radioresistant tumor , newer data have challenged this viewpoint, and radiation therapy is now considered to be a useful component of the therapeutic armamentarium for malignant melanoma. According to our results a single one-day sessions SRS with 35.0 Gy is sufficient to treat small and middle stage melanoma [21].
Conclusion
SRS is a non-invasive alternative to enucleation in the treatment of uveal melanoma with a high tumor control. One step LINAC based stereotactic radiosurgery with a single dose 35.0 Gy in conjunction with a mechanical immobilization system with four sutures according to our study is a highly effective method to treat amall and middle stage uveal melanoma.
According to our experience the dose of 35.0 Gy is not sufficient irradiation and may cause relapse only in patients with high volume tumors, over 0.6 cm3. By analyzing individual patient’s results of this study we conclude that this therapy is sufficient for small and intermediate tumors with the elevation not over 6 mm, resp. volume up to 0.4 cm3 according to individual stereotactic planning scheme of each patient as a single therapy procedure. According to our results one-day session SRS with 35.0 Gy is sufficient to treat small and middle stage melanoma.
References
- Furdova A, Slezak P, Chorvath M, Waczulikova I, Sramka M, Kralik G. No differences in outcome between radical surgical treatment (enucleation) and stereotactic radiosurgery in patients with posterior uveal melanoma. Neoplasma. 2010; 57: 377-381.
- Singh AD, Shields CL, Shields JA. Prognostic factors in uveal melanoma. Melanoma Res. 2001; 11: 255-263.
- Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005; 62: 1405-1411.
- Cohen VML, Carter MJ, Kemeny A, Radatz M, Rennie IG. Metastasis-free survival following treatment for uveal melanoma with either stereotactic radiosurgery or enucleation. Acta Ophthalmologica Scandinavica. 2003; 6: 383-388.
- Gragoudas ES, Lane AM, Munzenrider J, Egan KM, Li W. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans Am Ophthalmol Soc. 2002; 100: 43-48.
- Zehetmayer M. Stereotactic photon beam irradiation of uveal melanoma. Dev Ophthalmol. 2012; 49: 58-65.
- Dieckmann K, Dietmar G, Zehetmayer M, Bogner J, Georgopoulos M, Potter R. LINAC based stereotactic radiotherapy of uveal melanoma: 4 years clinical experience. Radiotherapy and Oncology. 2003; 67: 199-206.
- Dieckmann K, Dietmar G, Bogner J, Zehetmayer M, Petersch B, Chorvat M, et al. Optimizing LINAC-based stereotactic radiotherapy of uveal melanomas: 7 years clinical experience. Int J Radiation Oncology Biol Phys. 2006; 66: S47-S52.
- Meyer A, Lévy C, Blondel J, D'hermies F, Frau E, Schlienger P, et al. [Optic neuropathy after proton-beam therapy for malignant choroidal melanoma]. J Fr Ophtalmol. 2000; 23: 543-553.
- De Potter P, Shields CL, Shields JA, Cater JR, Tardio DT. Impact of enucleation versus plaque radiotherapy in the management of juxtapapillary choroidal melanoma on patient survival. Br J Ophthalmol. 1994; 78: 109–114.
- Marchini G, Gerosa M, Piovan E, Pasoli A, Babighian S, Rigotti M, et al. Gamma Knife stereotactic radiosurgery for uveal melanoma: clinical results after 2 years. Stereotact Funct Neurosurg. 1996; 66 Suppl 1: 208-213.
- Rennie I, Forster D, Kemeny A, Walton L, Kunkler I. The use of single fraction Leksell stereotactic radiosurgery in the treatment of uveal melanoma. Acta Ophthalmol Scand. 1996; 74: 558-562.
- Langmann G, Pendl G, Klaus-Müllner, Papaefthymiou G, Guss H. Gamma knife radiosurgery for uveal melanomas: an 8-year experience. J Neurosurg. 2000; 93 Suppl 3: 184-188.
- Mueller AJ, Talies S, Schaller UC, Horstmann G, Wowra B, Kampik A. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology. 2000; 107: 1381-1387.
- Zehetmayer M, Kitz K, Menapace R, Ertl A, Heinzl H, Ruhswurm I, et al. Local tumor control and morbidity after one to three fractions of stereotactic external beam irradiation for uveal melanoma. Radiother Oncol. 2000; 55: 135-144.
- Ghazi NG, Ketcherside CS, Sheehan J, Conway BP. Gamma knife radiosurgery for uveal melanoma ineligible for brachytherapy by the Collaborative Ocular Melanoma Study criteria. Open Access Surgery. 2008; 1: 21–24.
- Krema H, Somani S, Sahgal A, Xu W, Heydarian M, Payne D, et al. Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol. 2009; 93: 1172-1176.
- Mosci C, Mosci S, Barla A, Squarcia S, Chauvel P, Iborra N. Proton beam radiotherapy of uveal melanoma: Italian patients treated in Nice, France. Eur J Ophthalmol. 2009; 19: 654-660.
- Girkin CA, Comey CH, Lunsford LD, Goodman ML, Kline LB. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997; 104: 1634-1643.
- Henderson MA, Shirazi H, Lo SS, Mendonca MS, Fakiris AJ, Witt TC, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy in the treatment of uveal melanoma. Technol Cancer Res Treat. 2006; 5: 411-419.
- Furdova A, Strmen P, Waczulikova I, Chorvath M, Sramka M, Slezak P. One-day session LINAC-based stereotactic radiosurgery of posterior uveal melanoma. European Journal of Ophthalmology. 2012; 22: 226-235.