
Research Article
Austin J Radiat Oncol & Cancer. 2015; 1(3): 1014.
Abdominoperineal Resection is Associated with a Poor Outcome Compared with Anterior Resection for Rectal Cancer: A Meta-Analysis
Yen-Chien Lee1*, Chung-Cheng Hsieh2 and Jen- Pin Chuang1
1Department of Oncology, Tainan Hospital, Ministry of Health and Welfare, Tainan, Taiwan
2Department of Cancer Biology, University of Massachusetts Medical School, Worcester, UK
*Corresponding author: Yen-Chien Lee, Department of Oncology, Tainan Hospital, Ministry of Health and Welfare, ROC.No. 125, Jhongshan Rd, Tainan, Taiwan
Received: November 16, 2015; Accepted: December 01, 2015; Published: December 03, 2015
Abstract
Background: It’s controversies whether Anterior Resection (AR) is superior to Abdominoperineal Resection (APR) for rectal cancer. To solve this question, a meta-analysis was performed.
Methods: Studies published from 1990 to May 2014 evaluating outcomes after AR and APR for rectal cancer were identified by an electronic literature search. A meta-analysis was performed to compute Hazard Ratio (HR) along with 95% Confidence Intervals (CI). We extracted HRs of Disease-Free Survival (DFS) and Overall-Survival (OS) compared these two methods using a randomeffects model.
Results: Twenty four studies met the inclusion criteria, yielding a total of 21,221 patients. Pooled adjusted HRs were 1.276 (95% CI, 1.266 -1.287) for DFS and 1.130 (95% CI, 1.126-1.133) for OS that compared APR with AR. Sensitivity analyses were performed and APR was still associated with poor prognosis.
Conclusion: APR has been associated with poor prognosis compared with AR. While possible, a more radical operation, extralevator abdominoperineal resection should be considered.
Keywords: Rectal cancer; Meta-Analysis; Abdominoperineal resection; Disease free survival
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in males and second in females [1]. It is also one of the leading causes of cancer mortality worldwide, and rectal cancer accounts for 30 to 35% of these cases [1].
Miles, who introduced Abdominoperineal Resection (APR) in 1908, based his work on that the spread of tumors of the lower rectum occurred through the lymph nodes outside the levator ani muscles. APR was thought to address this problem. Anterior Resection (AR) was later introduced for proximal rectal cancers. Improvements in stapling technology over the following decades have resulted in an increase in the number of sphincter-saving operations [2]. The more important recent advance in rectal cancer surgery has been the Total Mesorectal Excision (TME). TME is defined as sharp pelvic dissection between the parietal and visceral planes of the pelvic fascia and it has been introduced since 1982 by Heald [3]. TME and precise perimesorectal plane dissection were introduced in all the major countries of Europe in 1995 [4]. Local recurrence rate can decrease to less than 10% for middle and lower rectal carcinomas with this procedure [5]. Following several workshops and operative demonstrations around the world, TME is now generally accepted as the standard procedure for rectal cancer [6].
Previously APR was thought to be able to eradicate border field than AR and achieve better outcomes in disease free survival or even overall survival. Others suggest otherwise [7-10]. It’s not clear if the worse outcome associated with APR is a result of surgical procedure or patient- and tumor-related factors. Many studies have tried to explicate these relationships. Possible reasons for high local recurrence of APR compared with AR include the higher incidence of inadequate margin [11] or lymph node involvement that may follow a different pattern in low rectal carcinomas. Very low cancers have a dual lymphatic drainage [12]. Tumor or rectal perforation are other important factors that might contribute to poorer outcomes in APR.
However, due to tumor nature and anatomic reason, further randomized-controlled trials would not be feasible to compare these two methods. This question has been further complicated by different neoadjuvant, adjuvant, and chemotherapy agents. Since prospective randomized studies comparing APR with AR are ethically unavailable, we try to answer this question from previous studies through metaanalysis. This is the first meta-analysis that attempts to answer this question.
Method
Search strategy and Selection criteria
We systematically searched Pub Med and Medlines for relevant articles published up to May12, 2014, with the following search terms, “anterior” and “rectal cancer, “abdominoperineal” and “rectal cancer” without language restriction. Additional search methods included manual review of the reference lists of relevant studies. Studies were included if the following were true: (1) they were published with extractable information on disease free survival (DFS) or overall survival (OS); (2) they were primary rectal cancer. Chemotherapy was not used in patients with rectal cancer during the late 1990s. Whether adjuvant was used or not are included as well. Studies before 1990 were excluded. Kaplan-Meier DFS or OS graph with three or fewer outcome events was considered as inadequate and was excluded. Only original articles were included; posters, abstracts, and conference reports were excluded.
Information on age and stage were extracted when available and taken into account in the analysis. Abdominoperineal excision was often involved positive circumferential resection margins [13] which has been associated with a poor prognosis [14]. Due to variable factors adjustments in each studies, if any one of stage, age or margin were included in original studies for adjustment, the HR were considered as multivariate analysis.
We assessed the methodologic quality of included studies using the Newcastle-Ottawa Scale [15] for determining the quality of individual cohort studies included in the meta-analysis.
Statistical analysis
We extracted HR by direct or indirect methods [16] based on the available information provided in the selected articles. If information for applying these methods was not available, a graph methods, which stratifies the published survival curve into nonoverlapping intervals to estimate the log HR, was used to extract the HR [16]. Summary HRs were obtained using the random-effect model. Forest plots were used to display the study-specific HRs and the summary estimates. Heretorgenetiy among the studies was tested with Q statistics. Analyses were conducted with Stata version 12.0 (Stata Corp, College Station, TX). Two-sided p values <0.05 were regarded as statistically significant.
Results
Literature search
The steps of our literature search are shown in (Figure 1). After reviewing titles and abstracts, 104 potential papers were extracted to review in full. Two papers [17,18] were from two different but overlap periods of the same hospital. Due to one paper provided information to overall survival and the other to disease-free survival without double weighting. Both of the papers were included.
Four articles were excluded due to language problem [19-22]. Four other articles were excluded due to unavailable in our setting [23-26]. Two of the four were from the same Chinese journal. As for the rest, one is excluded due to a short report [27]. One is excluded due to abdominosacral resection instead of abdominoperineal resection [28]. One is excluded due to use chi-square test for survival data [29]. Some paper with hazard ratio of local recurrence only [30]. Two paper is excluded due to less or equal to 3 events and was unable to extract hazard ratio from survival curve [31, 32]. Two were excluded due to more than 3 survival curve and unable to extract [33,34]. Others were excluded due to no extractable hazard ratio (HR) of DFS or OS. Methodological quality scores ranged from 4 to 9 on a scale of 10 (Table 1). With regard to the quality of the studies, 21 trials (87.5%) showed a high-quality score (6 points). The distribution of total scores for the 24 studies were as follows: 4 (1 study), 5 (2 studies), 6 (2 studies), 7 (4 studies), 8 (12 studies) and 9 (3 studies). Because most of the studies received a score of 6 or above and the standard criteria have not been established, we considered the studies to be of adequate quality for the analysis.
Studies
Representative-ness of the exposed cohort
Selection of the non-exposed cohort
Ascertainment of exposure: APR vs. LAR
Demonstration that outcome of interest not present at start
Study controls for initial staging and/or for an additional factor
Assessment of outcome
Was median follow-up 2.5 years or more?
Adequacy of follow-up (80%)
Total
Tuscano D [37]
—a
★
★
★
—
—
—
★
4
Holm T [36]
★b
★
★
★
★★
★
—
—
7
Nymann T [53]
★
★
★
★
—
—
—
★
5
Bozzetti F [38]
★
★
★
★
★★
★
★
—
8
Zaheer S[35]
★
★
★
★
★★
★
★
★
9
Wibe A [8]
★
★
★
★
★★
—
★
—
7
Law WL [39]
★
★
★
★
★★
★
★
—
8
Harling H [40]
★
★
★
★
★★
★
—
★
8
Nakagoe T [54]
★
★
★
★
★★
★
★
★
9
Marr R [41]
★
★
★
★
—
★
—
—
6
Haward RA [7]
★
★
★
★
★c
★
—
—
6
Chuwa EW [42]
★
★
★
★
★★
★
★
—
8
Kim NK [43]
★
★
★
★
★★
—
★
★
8
Ptok H [44]
★
★
★
★
★ ★
★
—
★
8
Saito N [45]
★
★
★
★
—
★
★
★
7
den Dulk M [46]
★
★
★
★
★★
★
★
★
9
Ferenschild FT [47]
★
★
★
★
★★
★
★
—
8
Kim JS [17]
★
★
★
★
★★
—
★
—
7
Anderin C [48]
★
★
★
★
★★
★
★
—
8
Silberfein EJ [49]
★
★
★
★
★★
★
★
—
8
Chambers W [50]
★
★
★
★
—
—
★
—
5
Lange MM [51]
★
★
★
★
★★
—
★
★
8
Omidvari S [52]
★
★
★
★
★★
★
★
—
8
Kim JC [18]
★
★
★
★
★★
—
★
—
7
The first score, representativeness of the exposed cohort, was scored as positive where the study was considered truly or somewhat representative of the general population in the location of the study. Studies on selected groups or where there was no description if the cohort were scored as negative. The second score, selection of the non-exposed cohort, was scored as positive if the population was selected and then each subject was identified as either exposed or non-exposed, so that the non-exposed cohort was drawn from the same community as the exposed cohort. The third score, ascertainment of exposure, related to operation methods in our analysis. The fourth score, demonstration that the outcome of interest was not present at start of the study, was scored as positive as operation outcome was not presented initially. The fifth score, comparability of the cohorts on the basis of design or analysis, was scored according to whether the analysis controlled for initial staging and/or for an additional factor (e.g. marginal status, age, or others). The sixth score, assessment of outcome, was scored positively if one of outcomes or start time were defined clearly initially. The seventh score was was follow-up long enough for outcomes to occur. It was decided that a median follow-up of greater than 2.5 years would be adequate as most colon cancer recurred within 2 years [9]. The eighth and final score, adequacy of follow up of cohorts was scored positively if the follow-up was complete or the subjects lost to follow up were less than 20%. The highest possible total score was 9. The distribution of total scores for the 24 studies were as follows: 4 (1 study), 5 (2 studies), 6 (2 studies), 7 (4 studies), 8 (12 studies) and 9 (3 studies). A) Tuscano D didnt specify whether these 34 patients represent all these group of patients or not. B) This study combined two different time periods of study groups. C) This study didnt adjust for stage information but adjust for other factors.
Table 1: Methodologic quality of studies, based on the Newcastle-Ottawa scale (N = 24).
Study characteristics
We identified 24 eligible studies [7,8,17,18,35-54] published from 1990 to 2014 (Table 2a). There were 21,221 patients included in these studies (Table 2b). All were retrospective cohort studies except one matched retrospective cohort study. [18] Fifteen studies were from Europe, two studies from United States and seven studies from Asia. Patients received variable radiation and chemotherapies. The predominant chemo radiation therapy regimens and adjuvant chemotherapies were 5-fluorouracil-based.
HRs of data from one article was calculated by the direct methods, 16 by the indirect methods, and seven by the graph method. The median age of the patients was around 60 years, and the majority of the patients were men. Most studies calculated the start points from the date of primary treatment (surgery) except one [7] that started from the diagnosis date (Table 3). Eight out of the 24 studies did not provide information on HRs of disease-free survival or overall survival.
Study
Year
country
Design
No. of
institutes
Male (%)
location
Radiation
Chemoradiation regimen
Adjuvant chemotherapy
Tuscano D [37]
1992
Italy
Retrospective
Single
67
?7.5cm
No
No
No
Holm T[36]
1995
Sweden
Retrospective
Multiple
59
?25cm
47.7 vs. 49.6%
No
No
Nymann T [53]
1995
Denmark
Retrospective
single
63
?18cm
No
No
No
Bozzetti F [38]
1996
Italy
Retrospective
Single
59
?8cm
NA
NA
NA
Zaheer S [35]
1998
US
Retrospective
Single
61
NA
No
No
No
Wibe A [8]
2004
Norway
Retrospective
47
58
?12cm
6 vs. 16%
No
No
Law WL [39]
2004
Hong Kong
Retrospective
Single
61
?12cm
7.1 vs. 45
5FU
44.4 vs. 41
II, III
Harling H [40]
2004
Denmark
Retrospective
Multiple
NA
?15cm
34%
NA
NA
Nakagoe T [54]
2004
Japan
Retrospective
Single
64
NA
No
No
III, 5FU
Marr R [41]
2005
UK
Retrospective
Single
56
NA
No
No
11.9%
Haward RA [7]
2005
UK
Retrospective
Multiple
61
NA
NA
NA
NA
Chuwa EW [42]
2006
Singapor
Retrospective
Single
58
?10cm
No
No
No
Kim NK [43]
2006
Korea
Retrospective
single
70
NA
all
5FU_LV
All, 5FU_LV
Ptok H [44]
2007
German
Retrospective
75
61
?8 cm
NA
NA
49.7 vs. 53.4
Saito N [45]
2009
Japan
Retrospective
Single
70
?5 cm
No
T3 (36.4%)
III 5FU_LV, UFUR, others
den Dulk M [46]
2009
Europe
Retrospective
Multiple
66
NA
Variable
5FU_LV
5FU_LV
Ferenschild FT [47]
2009
Netherlands
Retrospective
Single
54
?15cm
Variable
Variable
No
Kim JS [17]
2009
Korea
Retrospective
Single
72
?6cm
Yes
5FU_LV
NA
Anderin C [48]
2010
Sweden
Retrospective
8
57
?6cm
73 vs71%
NA
NA
Silberfein EJ [49]
2010
US
Retrospective
Single
57
NA
88%
5FU, capecitabine
II or III, 5FU_LV
Chambers W [50]
2010
UK
Retrospective
Single
68
NA
63 vs. 59.5%
NA
3.7 vs 26.2%
Lange MM [51]
2013
Netherlands
Retrospective
2
61
?12cm
Yes
5FU_LV
5FU_LV, levamisole, or capecitabine
Omidvari S [52]
2013
Iran
Retrospective
Single
58
?12cm
84.4 vs. 71.4%
5FU_LV, capecitabine, oxaliplatin
5FU_LV, capecitabine, oxaliplatin, irinotecan
57.9 vs 42.1%
Kim JC [18]
2013
Korea
Retrospective, matched
Single
59
?6cm
39 vs 13%
5FU-LV or capecitabine
III, II with poor prognostic factor
Table 2a: Studies of compared low anterior resection with abdominoperineal resection.
Source
Median follow up (mo.)
Median age
stage
Methods
TME
AR
APR
DFS_HR_U
DFS_HR_M
OS_HR_U
OS_HR_M
Adjuvstagea
Adjuagea
Adju
margina
25b
60
?III
Direct
Yes
13
11
NA
NA
1.21
NA
-
-
-
Holm T
NA
68 vs. 69c
?III
Indirect
NA
470
664
NA
NA
NA
1.14
Yes
Yes
Yes
Nymann T
NA
68.7 vs. 64.9c
?III
Graph
NA
74
101
0.64
NA
NA
NA
-
-
-
Bozzetti F
77
64
?III
Indirect
Yes
93
257
NA
NA
NA
0.72
Yes
Yesd
Yes
Zaheer S
5.6y
67
all
Graph
Yes
272
169
NA
NA
1.20
NA-
-
-
-
Wibe A
44
69
?III
Indirect
Yes
1315
821
NA
NA
1.86
1.3
Yes
Yes
Yes
Law WL
35.4
67
all
Indirect
Yes
419
69
NA
1.94
NA
NA
Yes
Yes
Yes
Harling H
NA
NA
?III
Indirect
V
2199
1263
NA
NA
1.26
NA
-
-
-
Nakagoe T
47.4
66
?III
Indirect
Yes
116
91
1.23
0.74
NA
NA
Yes
Yes
Yes
Marr R
NA
NA
NA
Graph
Yes
355
181
1.47
NA
NA
NA
-
-
-
Haward RA
NA
NA
NA
Indirect
NA
1535
1986
NA
NA
NA
1.10
No
Yes
No
Chuwa EW
38
65
?III
Indirect
Yes
547
93
1.23
0.88
NA
NA
Yes
Yes
NA
Kim NK
39.4
55
?III
Indirect
Yes
41
56
NA
NA
NA
2.86
yes
No
Yes
Ptok H
NA
66
?III
Indirect
Yes
601
956
NA
1.26
NA
NA
Yes
No
Yes
Saito N
40 vs. 58c
57 vs.59c
?III
Graph
Yes
132
70
1.65
NA
1.22
NA
-
-
-
den Dulk M
5.4y
NA
?III
Indirect
V
2280
1353
NA
1.31
NA
1.17
Yese
Yesf
Yes
Ferenschild FT
3.6y
69g
?IIIh
Indirect
Yes
145
65
NA
NA
NA
0.74
Yese
Yes
Yes
Kim JS
47.7
NA
?III
Graph
Yes
72
50
1.32
NA
NA
NA
-
-
-
Anderin C
6.4y
67vs. 72c
?III
Indirect
Yes
113
438
NA
NA
1.50
1.19
Yes
Yes
Yes
95
57
?III
Indirect
Yes
176
128
NA
NA
1.79
1.75
Yes
Yes
No
Chambers W
4.8y
64
?III
Graph
NA
81
42
2.38
NA
NA
NA
-
-
-
Lange MM
92
71g
?III
Indirect
Yes
259
107
NA
NA
1.11
1.45
Yes
Yes
No
Omidvari S
37
57h
Alli
Graphj
Yes
96
42
NA
NA
1.81
NA
-
-
-
Kim JC
76 vs. 84c
54g
?III
Indirect
Yes
402
402
NA
NA
NA
0.994
Yes
Yes
NA
Abbreviation: NA, not available; TME, total mesorectal excision; DFS_HR_U, univariate of hazard ratio for disease-free survival, APR/AR; DFS_HR_M, multivariate of hazard ratio for disease-free survival, APR/AR; OS_HR_U, univariate of hazard ratio for overall-survival, APR/AR; OS_HR_M, multivariate of hazard ratio for overall-survival, APR/AR; V, variable.
aDFS_HR_M or OS_HR_M adjusted for stage, age or margin; bMean follow up; bAR vs. APR; cage is excluded while p-value less than 0.2 during univariate analysis and then not included in Cox model; donly adjusted for lymph node status; epropensity score; fMean age; g4.8% liver metastasis not found initially but found during operation; h15% of stage IV; i12% laparoscopic and 88% open approach
Table 2b: Patient characteristics of studies.
Studies
Start time
Disease-free survival
Overall survival
Tuscano D [37]
NA
NA
NA
Holm T [36]
NA
Recurrence within the radiation target area defined as local and outside this area as distant metastasis
NA
Nymann T [53]
NA
NA
NA
Bozzetti F [38]
Surgery date
Pelvic recurrence, distant metastases, or second primary
Occurrence of death, or to the last follow-up assessment available for living patients
Zaheer S [35]
Primary treatment
Date of first recurrence
Death
Wibe A [8]
NA
NA
NA
Law WL [39]
Surgery date
Histologically proven or radiologically evident disease with subsequent clinical progression
NA
Harling H [40]
Surgery date
NA
Death or when censored
Nakagoe T [54]
Surgery date
Date of first recurrence
NA
Marr R [41]
NA
Cancer-specific survival, died of noncancer-related illness were censored from further analysis from the time of death
NA
Haward RA [7]
Diagnosis date
NA
Death or when censored
Chuwa EW [42]
Date of primary treatment
Date of first recurrence-local, systemic, or both
Date of death
Kim NK [43]
NA
NA
NA
Ptok H [44]
Surgery date
A local and/or systemic recurrence
A new tumor , either histologically or an imaging
Death of the patients, irrespective of its cause
Saito N [45]
Surgery date
NA
NA
den Dulk M [46]
Surgery date
Death due to rectal cancer
NA
Ferenschild FT [47]
Surgery date
NA
NA
Kim JS [17]
NA
NA
NA
Anderin C [48]
Surgery date
NA
Time of death or the end of follow-up
Silberfein EJ [49]
Surgery date
NA
NA
Chambers W [50]
NA
NA
NA
Lange MM [51]
NA
NA
NA
Omidvari S [52]
Date of initial treatment
Any type of treatment failure
Death from any reason or the last follow-up
Kim JC [18]
NA
NA
NA
Table 3: Methodologic definitions.
Disease free survival and overall survival
As univariate analysis (Table 2b), six out of 24 had information of disease-free survival, the pooled HR compared APR with AR was 1.311 (95% CI, 1.272-1.352). There was significant heterogeneity (p<0.001, Figure 1a). HRs from nine studies on OS was 1.264 (95% CI, 1.257-1.270), also with significant heterogeneity (p<0.001, Figure 2a).
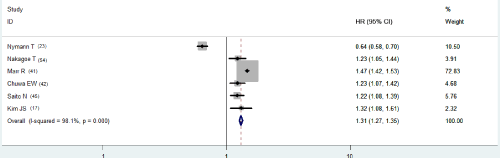
Figure 1a: Univariate HR of DFS.
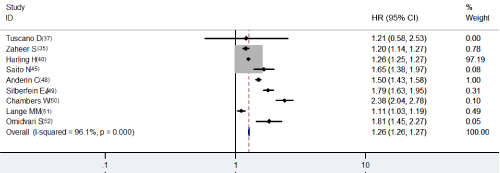
Figure 2a: Univariate HR of OS.
As for multivariate HR of DFS, studies were adjusted different for stage, age and margin (Table 2b). Combining five papers HRs on DFS adjusting variable for stage, ages and margin, [39, 42, 44, 46, 54] the pooled HR comparing APR with AR was 1.276 (95% CI, 1.266-1.287), again with significant heterogeneity (p<0.001, Figure 1b). Multivariate HRs of OS [7,8,17,18,36,38,43,47-49,51] adjusted variable for stage (eight studies), age (eight studies) and margin (five studies) was 1.130 (95% CI, 1.126-1.133) (Table 2b). There was a statistically significant heterogeneity among studies (p<0.001, Figure 2b).
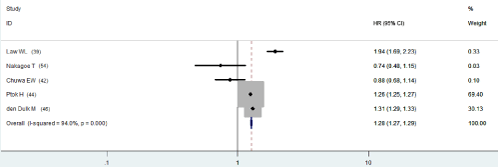
Figure 1b: Multivariate HR of DFS.
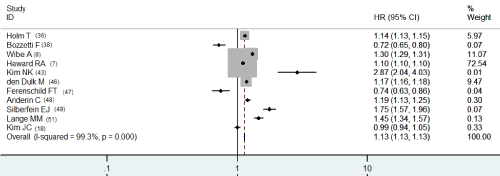
Figure 2b: Multivariate HR of OS.
Sensitivity analyses
Potential sources of heterogeneity include tumor location of the rectal cancer, geographical region where the study was conducted and year of the treatment. Also, age was adjusted for with different methods. To assess if any of these factors causing heterogeneity, subgroup analysis were performed within stratum of the relevant study features. Subgroup analyses were performed for tumor located within 12cm of anal verge. Subgroups for different regions of Europe and Asia were analysis and for those received operation within 10 years (2004) as well.
Heterogeneity was equally evident in all strata except DFS in studies conducted in Asia and for tumor within 12cm anal verge (data not shown). However, the studies numbers were small. Nonetheless, APR continues to show a poor prognosis as compared with AR in different subgroups.
Discussion
Cancers of the lower rectum have a less favorable oncologic outcome compared with those situated at upper locations [8, 55- 57]. Lower rectal carcinomas had a twice chance of circumferential margin involvement compared to tumors situated over 5 cm from anal verge (26.5% v 12.6%). After chemo radiation therapy, AR might be performed as well. In lower rectal carcinoma, three times more positive margins were present in patients operated by APR than AR (30.4% v 10.7%) [58]. In the late nineteenth century, APR was the standard operation for lower rectal cancer. Due to the development of stapler technique, AR was later more widely adopted. Previously APR was thought to have better survival due to the wider dissection [37]. However, later studies showed that APR was associated with poor survival due to involvement of more positive margins [13,48] or perforation, [48] higher local recurrence rates, and poorer survival of low rectal cancer [58]. Even after adjusting for margins and perforation, APR was still associated with a poor prognosis [44]. Among the studies included in our analysis, most of the HRs for OS were adjusted for stage, age and margin and APR was still associated with a poor prognosis. The median tumor level located from anal verge also lower in the APR group than in the AR group [48]. In early 1990s, TME was not widely adopted but now it was current standard of treatment. TME cannot always be performed [9] because of the presence of a large tumor around this lower level. Visualization and access at the floor of the pelvis are limited. Nevertheless 18 out of 24 (75%) studies stated that they had performed TME on all study patients with 2 studies of variable performing TME.
In our analysis, we showed that APR was associated with poor disease free and overall survival even after adjusted for age, margin and stage. Though we found a significant heterogeneity in the pooled disease free survival or overall survival of APR compared with AR, all the analysis and most of the subgroup analyses showed the poor prognosis associated with APR while compared with AR. The heterogeneity might due to patient racial composition or difference in age adjustment. Holm T et al., [36] indicated that they had adjusted for age without a detailed description. Some studies adjusted for age with variable cut points [38] or with propensity score [8]. One study adjusted for age at cut point of 65 year-old [42] while others at 80 year-old [47] or 72 year-old [48]. Also, variable definitions of clear margin were used. The proximal, distal, and circumferential resection margins free from tumor instead of tumor involvement within 1 mm of the circumferential resection margin (CRM positive) were used by Anderin C et al., [48] while others did not provide a clear definition.[36] Radiation therapy has been a standard care for T3 rectal cancer [59]. Since the introduction of radiotherapy, local recurrence has been reduced from 11.4% to 6.1% [60]. Radiotherapy has been considered as standard therapy for stage II and III rectal cancer. However, radiation therapy has only shown a marginal effect in improvement. Due to the lack of strong evidence specifically for rectal cancer, support for the use of adjuvant chemotherapy in patients with rectal cancer is generally extrapolated from the data available for colon cancer [61].
P. How et al., [10] has published a review article of “a systematic review of cancer related patient outcomes after anterior resection and abdominoperineal excision for rectal cancer in the total mesorectal excision era” in 2011. They suggested that tumours treated by APR were lower and more locally advanced. They suggested that surgical technique itself for the tradition APR was also inadequate and resulted in poor outcomes. The results of our meta-analysis support their conclusion as well.
In recent years, a more extended approach, later called the extralevator abdominoperineal resection, has been propose by T. Holm [62]. Review article suggested that extended APR such as cylindrical resection was associated with a lower rate of radial margin involvement and improved local control rate compared to conventional APR [63]. A meta-analysis has confirmed this finding [64].
Up to 90% of patients undergo sphincter-preserving surgery will subsequently have a change in bowel habit, ranging from increased bowel frequency to fecal incontinence or evacuatory dysfunction. These have been called anterior resection syndrome [65]. An uncertain preference for one or the other surgical procedure cannot be discerned, particularly a prospective study [66] and a meta-analysis [67] on the quality of life following APR vs. AR had failed to show a superiority of AR.
Some might argue that the surgical indications for APR were different from anterior resection. Decision was based on the location of the tumor. Difference in tumor locations could also reflect a difference in tumor biology in term of spread. To answer this theory, one should compare APR vs anterior resection in upper 2/3 rectal cancer which might seem impossible due to unethical. However, as we could see that 7 out of 24 inclusions studies were from lower rectum (8cm) (Table 2A). Among them 3 papers were available for univariate OS analysis. It still showed that APR were worse than LAR (HR 1.509, 95% CI, 1.439-1.582) but non-significant after adjusted for age or margin (HR 0.954, 95% CI, 0.761-1.193) (data not shown).
Rectal cancer treatment was a rather complex issue. Surgical options including LAR or APR were performed by tumor location and the extent of tumor invasion. Despite of several trials have been done to compare the long term outcome of this two surgical options, there was still no consensus nor guideline to follow. The major reasons may be small size in each trial or dispersed distribution of rectal cancer in different patients or even each surgeon’s personal favor in certain surgical procedure. In order to solve these problems, meta-analysis could provide a more robust evidence to this important clinical issue.
Further study might be needed whether the poor prognosis is due to rupture during the operation. Due to the poor prognosis of APR compared with AR, extralevator abdominoperineal resection [64] might be another choice while APR were deem unsuitable.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61: 69-90.
- Inoue Y, Kusunoki M. Resection of rectal cancer: a historical review. Surg Today. 2010; 40: 501-506.
- Heald RJ, Husband EM, Ryall RD . The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982; 69: 613-616.
- Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg. 1995; 82: 1297-1299.
- Piso P, Dahlke MH, Mirena P, Schmidt U, Aselmann H, Schlitt HJ. Total mesorectal excision for middle and lower rectal cancer: a single institution experience with 337 consecutive patients. J Surg Oncol. 2004; 86: 115-121.
- Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998; 133: 894-899.
- Haward RA, Morris E, Monson JR et al., The long term survival of rectal cancer patients following abdominoperineal and anterior resection: results of a population-based observational study. Eur J Surg Oncol 2005; 31: 22-28.
- Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O. Norwegian Rectal Cancer Group . Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004; 47: 48-58.
- Heald RJ, Smedh RK, Kald A, Sexton R, Moran BJ. Abdominoperineal excision of the rectum--an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum. 1997; 40: 747-751.
- How P, Shihab O, Tekkis P et al., A systematic review of cancer related patient outcomes after anterior resection and abdominoperineal excision for rectal cancer in the total mesorectal excision era. Surg Oncol 2011; 20: 149-155.
- Tilney HS, Tekkis PP, Sains PS, Constantinides VA, Heriot AG. Association of Coloproctology of Great Britain and Ireland . Factors affecting circumferential resection margin involvement after rectal cancer excision. Dis Colon Rectum. 2007; 50: 29-36.
- Suzuki K, Muto T, Sawada T. Prevention of local recurrence by extended lymphadenectomy for rectal cancer. Surg Today. 1995; 25: 795-801.
- Den Dulk M, Marijnen CA, Putter H, Rutten HJ, Beets GL, Wiggers T. Risk factors for adverse outcome in patients with rectal cancer treated with an abdominoperineal resection in the total mesorectal excision trial. Ann Surg. 2007; 246: 83-90.
- Kelly SB, Mills SJ, Bradburn DM, Ratcliffe AA, Borowski DW. Northern Region Colorectal Cancer Audit Group . Effect of the circumferential resection margin on survival following rectal cancer surgery. Br J Surg. 2011; 98: 573-581.
- GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2014.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998; 17: 2815-2834.
- Kim JS, Hur H, Kim NK, Kim YW, Cho SY, Kim JY. Oncologic outcomes after radical surgery following preoperative chemoradiotherapy for locally advanced lower rectal cancer: abdominoperineal resection versus sphincter-preserving procedure. Ann Surg Oncol. 2009; 16: 1266-1273.
- Kim JC, Yu CS, Lim SB, Kim CW, Kim JH, Kim TW. Abdominoperineal resection and low anterior resection: comparison of long-term oncologic outcome in matched patients with lower rectal cancer. Int J Colorectal Dis. 2013; 28: 493-501.
- Kneist W, Heintz A, Wolf HK, Junginger T. Total excision of the mesorectum in cancer of the lower and middle rectum. Oncological and functional results. Chirurg. 2003; 74: 125-131.
- Graf W, Påhlman L, Enblad P, Glimelius B. Anterior versus abdominoperineal resections in the management of mid-rectal tumours. Acta Chir Scand. 1990; 156: 231-235.
- Palasciano N, Volpi A, Ricci P, Caputi Jambrenghi L, Orlando S, De Pasquale G . Effect of the resection margin and the extrarectal spread on the recurrence following 2 types of surgical procedures in rectal cancer. Rev Esp Enferm Dig. 1991; 80: 28-32.
- Boschi L, Lecce F, Bazzocchi F, Del Gaudio A. Surgical treatment of rectal cancer: comparison of anterior resection and abdominoperineal excision. Minerva Chir. 1995; 50: 831-834.
- Li CS1, Wan DS, Pan ZZ, Zhou ZW, Chen G, Wu XJ. Multivariate prognostic analysis of patients with low and middle rectal cancer after curative resection. Ai Zheng. 2006; 25: 587-590.
- He WJ, Wang L, Hu H, Kang SY, Qian HX, Xu FM. Correlation of invasion, metastasis, and prognosis in low and middle rectal cancer. Ai Zheng. 2002; 21: 1222-1225.
- Pakkastie TE, Luukkonen PE, Järvinen HJ. Anterior resection controls cancer of the rectum as well as abdominoperineal excision. Eur J Surg. 1995; 161: 833-839.
- Fick TE, Baeten CG, von Meyenfeldt MF, Obertop H. Recurrence and survival after abdominoperineal and low anterior resection for rectal cancer, without adjunctive therapy. Eur J Surg Oncol. 1990; 16: 105-108.
- Wolmark N, Fisher B. An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes' B and C rectal carcinoma. A report of the NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. Ann Surg. 1986; 204: 480-489.
- Bebenek M, PudeΕko M, Cisarz K, Balcerzak A, Tupikowski W, Wojciechowski L. Therapeutic results in low-rectal cancer patients treated with abdominosacral resection are similar to those obtained by means of anterior resection in mid- and upper-rectal cancer cases. Eur J Surg Oncol. 2007; 33: 320-323.
- Amato A, Pescatori M, Butti A. Local recurrence following abdominoperineal excision and anterior resection for rectal carcinoma. Dis Colon Rectum. 1991; 34: 317-322.
- Rullier E, Laurent C, Carles J, Saric J, Michel P, Parneix M. Local recurrence of low rectal cancer after abdominoperineal and anterior resection. Br J Surg. 1997; 84: 525-528.
- Do L, Syed N, Puthawala A, Azawi S, Shbeeb I, Gong IY. Low-lying rectal cancer with anal canal involvement: abdominoperineal or low anterior resection after neoadjuvant chemoradiotherapy. Gastrointest Cancer Res. 2011; 4: 90-95.
- Tschmelitsch J, Kronberger P, Prommegger R, Reibenegger G, Glaser K, Bodner E. Survival and local recurrence after anterior resection and abdominoperineal excision for rectal cancer. Eur J Surg Oncol. 1995; 21: 640-643.
- Okaro AC, Worthington T, Stebbing JF, Broughton M, Caffarey S, Marks CG. Curative resection for low rectal adenocarcinoma: abdomino-perineal vs anterior resection. Colorectal Dis. 2006; 8: 645-649.
- Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009; 249: 236-242.
- Zaheer S, Pemberton JH, Farouk R, Dozois RR, Wolff BG, Ilstrup D. Surgical treatment of adenocarcinoma of the rectum. Ann Surg. 1998; 227: 800-811.
- Holm T, Rutqvist LE, Johansson H, Cedermark B. Abdominoperineal resection and anterior resection in the treatment of rectal cancer: results in relation to adjuvant preoperative radiotherapy. Br J Surg. 1995; 82: 1213-1216.
- Tuscano D, Catarci M, Saputelli A, Gaj F, Gossetti F, Guadagni S. Low anterior resection versus abdominoperineal excision: a comparison of local recurrence after curative surgery for "very low" rectal cancer. Surg Today. 1992; 22: 313-317.
- Bozzetti F, Mariani L, Miceli R, Doci R, Montalto F, Andreola S. Cancer of the low and middle rectum: local and distant recurrences, and survival in 350 radically resected patients. J Surg Oncol. 1996; 62: 207-213.
- Law WL, Chu KW. Abdominoperineal resection is associated with poor oncological outcome. Br J Surg. 2004; 91: 1493-1499.
- Harling H, Bülow S, Kronborg O, Møller LN, Jørgensen T. Danish Colorectal Cancer Group. Survival of rectal cancer patients in Denmark during 1994-99. Colorectal Dis. 2004; 6: 153-157.
- Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005; 242: 74-82.
- Chuwa EW, Seow-Choen F. Outcomes for abdominoperineal resections are not worse than those of anterior resections. Dis Colon Rectum 2006; 49: 41-49.
- Kim NK, Baik SH, Seong JS, Kim H, Roh JK, Lee KY. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: Impact of postirradiated pathologic downstaging on local recurrence and survival. Ann Surg. 2006; 244: 1024-1030.
- Ptok H, Marusch F, Kuhn R, Gastinger I, Lippert H. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol. 2007; 33: 854-861.
- Saito N, Sugito M, Ito M, Kobayashi A, Nishizawa Y, Yoneyama Y. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg. 2009; 33: 1750-1756.
- den Dulk M, Putter H, Collette L, Marijnen CA, Folkesson J, Bosset JF. The abdominoperineal resection itself is associated with an adverse outcome: the European experience based on a pooled analysis of five European randomised clinical trials on rectal cancer. Eur J Cancer. 2009; 45: 1175-1183.
- Ferenschild FT, Dawson I, de Wilt JH et al., Total mesorectal excision for rectal cancer in an unselected population: quality assessment in a low volume center. Int J Colorectal Dis 2009; 24: 923-929.
- Anderin C, Martling A, Hellborg H, Holm T. A population-based study on outcome in relation to the type of resection in low rectal cancer. Dis Colon Rectum. 2010; 53: 753-760.
- Silberfein EJ, Kattepogu KM, Hu CY, Skibber JM, Rodriguez-Bigas MA, Feig B. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010; 17: 2863-2869.
- Chambers W, Khan A, Waters R, Lindsey I, George B, Mortensen N. Examination of outcome following abdominoperineal resection for adenocarcinoma in Oxford. Colorectal Dis. 2010; 12: 1192-1197.
- Lange MM, Martz JE, Ramdeen B, Brooks V, Boachie-Adjei K, van de Velde CJ. Long-term results of rectal cancer surgery with a systematical operative approach. Ann Surg Oncol. 2013; 20: 1806-1815.
- Omidvari S, Hamedi SH, Mohammadianpanah M, Razzaghi S, Mosalaei A, Ahmadloo N. Comparison of abdominoperineal resection and low anterior resection in lower and middle rectal cancer. J Egypt Natl Canc Inst. 2013; 25: 151-160.
- Nymann T, Jess P, Christiansen J. Rate and treatment of pelvic recurrence after abdominoperineal resection and low anterior resection for rectal cancer. Dis Colon Rectum. 1995; 38: 799-802.
- Nakagoe T, Ishikawa H, Sawai T, Tsuji T, Tanaka K, Hidaka S. Survival and recurrence after a sphincter-saving resection and abdominoperineal resection for adenocarcinoma of the rectum at or below the peritoneal reflection: a multivariate analysis. Surg Today. 2004; 34: 32-39.
- Enker WE, Havenga K, Polyak T, Thaler H, Cranor M. Abdominoperineal resection via total mesorectal excision and autonomic nerve preservation for low rectal cancer. World J Surg. 1997; 21: 715-720.
- Jatzko GR, Jagoditsch M, Lisborg PH, Denk H, Klimpfinger M, Stettner HM. Long-term results of radical surgery for rectal cancer: multivariate analysis of prognostic factors influencing survival and local recurrence. Eur J Surg Oncol. 1999; 25: 284-291.
- Lopez-Kostner F, Lavery IC, Hool GR, Rybicki LA, Fazio VW. Total mesorectal excision is not necessary for cancers of the upper rectum. Surgery. 1998; 124: 612-617.
- Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P. Dutch Colorectal Cancer Group; Pathology Review Committee. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005; 23: 9257-9264.
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990; 264: 1444-1450.
- Påhlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjödahl R. The Swedish rectal cancer registry. Br J Surg. 2007; 94: 1285-1292.
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004; 350: 2343-2351.
- Holm T, Ljung A, Häggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007; 94: 232-238.
- Stelzner S, Koehler C, Stelzer J, Sims A, Witzigmann H. Extended abdominoperineal excision vs. standard abdominoperineal excision in rectal cancer--a systematic overview. Int J Colorectal Dis. 2011; 26: 1227-1240.
- Huang A, Zhao H, Ling T, Quan Y, Zheng M, Feng B. Oncological superiority of extralevator abdominoperineal resection over conventional abdominoperineal resection: a meta-analysis. Int J Colorectal Dis. 2014; 29: 321-327.
- Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012; 13: e403-408.
- Schmidt CE, Bestmann B, Küchler T, Longo WE, Kremer B. Prospective evaluation of quality of life of patients receiving either abdominoperineal resection or sphincter-preserving procedure for rectal cancer. Ann Surg Oncol. 2005; 12: 117-123.
- Cornish JA, Tilney HS, Heriot AG, Lavery IC, Fazio VW, Tekkis PP. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. 2007; 14: 2056-2068.