
Research Article
Austin J Radiat Oncol & Cancer. 2016; 2(1): 1016.
Partial Breast Irradiation for Boost Using Image-Guided Brachytherapy: Florida Community Experience and Review of the Literature
Kuruvilla A¹*, Paryani S¹, Paryani N¹, Simmons D¹, Shah N¹, Caudill J², Stillword J² and Sullivan JW³
1First Radiation & Oncology Group, Cancer Center of Putnam, Palatka, Florida, USA
2General Surgery, Putnam Community Medical Center,USA
3Medical Oncology, Cancer Center of Putnam, Palatka, Florida, USA
*Corresponding author: Kuruvilla A, Cancer Center of Putnam, 600 Zeagler Drive, Palatka, Florida, USA
Received: May 07, 2016; Accepted: May 27, 2016; Published: May 30, 2016
Abstract
Purpose: To evaluate the feasibility of implementing non-invasive, imageguided breast brachytherapy (NIBB) in a community setting.
Materials & Methods: We reviewed our experience of treating 91 patients with early stage breast cancer since 2011. All patients were seen at a community cancer center serving about 100,000 people. Of the total number of patients seen, 7 elected to undergo modified radical mastectomy. 71 women underwent breast conserving surgery (BCS) followed by whole breast radiation therapy (WBRT) to a total dose of 45Gy along with a NIBB boost of 16Gy. 13 patients received WBRT with either an electron beam or photon boost. Most patients received NIBB at the initiation of their therapy followed by WBRT. All patients were followed at our center for toxicity and cosmesis by a single physician.
Results: All patients completed therapy as prescribed. Grade 2 or higher acute skin toxicity was observed in less than 10% of our patients. There was no grade 4 toxicity. Cosmesis at minimum of 6 months follow-up was judged to be excellent or good in 90% of women.
Conclusion: NIBB can be delivered safely and effectively in a community setting. The results presented here are the first single center, community results using NIBB. The results compare favorably with previously reported results.
Keywords: Brachytherapy; NIBB; Breast conserving surgery; Low dose rate; High dose rate
Introduction
This article details the First Radiation & Oncology Group’s experience at the Cancer Center of Putnam, Palatka, Florida. Whenever feasible, Non Invasive Breast Brachytherapy (NIBB) has been used as the preferential breast tumor bed boost technique for patients receiving irradiation after Breast Conserving Surgery (BCS) since initial implementation of this technology in 2011. At this center we have not yet offered NIBB as monotherapy following BCS.
Multiple randomized studies support the use of whole breast with external beam radiation therapy along with a boost after BCS [1,2]. Boost therapy has been reported to improve local control after lumpectomy and whole breast radiotherapy. Initial attempts to define a boost target involved scar-based palpation, but this practice has diminished with realization that the scar often did not correlate with the tumor bed. Interstitial Low Dose Rate (LDR) brachytherapy boosts, initially felt to be a promising technique for accurate localization of the boost volume, have also been decreasing in popularity, due to the availability of less invasive boosts [3]. The current standard of care is to boost with electrons or photons, and the modality chosen is usually dictated by the location and depth of the tumor bed [4]. Photon irradiation may be either via 3-dimensional conformal radiation therapy or intensity modulated radiation therapy. In some patients, it can be challenging to identify the tumor bed, particularly when surgical clips have not been placed. This difficulty is compounded in patients who undergo neoadjuvant chemotherapy. Attempts to localize the tumor bed on treatment planning Computed Tomography (CT), which is often inaccurate, result in unnecessarily large volumes of normal breast being targeted as part of the boost. A more novel technique involves NIBB, which utilizes High Dose Rate (HDR) brachytherapy incorporated with real time mammography for target localization and delineation.
All of the studies currently reported using NIBB were conducted at multi-center trials or academic facilities [5,6]. This is the first study examining the results of patients treated in a community setting serving a population of about 100,000 people.
Materials and Methods
Since implementation of NIBB in 2011, 91 breast cancer patients have been referred to the Cancer Center of Putnam. Of these, 84 (92%) chose BCS. 71 (78%) of these patients were boosted using NIBB.
Our protocol has been to treat the tumor bed with 4 fields. It takes a physicist approximately 30 minutes to perform daily Quality Assurance (QA) on the HDR. As such, we allocate a 60 minute slot per patient and treating all 4 fields is generally accomplished in this window. Once the patient is in the room, set up, including compression and images acquisition, is accomplished within 15 minutes. The physician is called to select the appropriate applicator and positioning coordinates. Treatment planning mammogram is correlated with pre-surgical mammogram (Figure 1 and 2) and referencing margin proximity in all dimensions from the pathology report. This typically takes the physician less than 5 minutes. The physician also verifies the plate separation required and from the Surface to Center Dose (SCD) the appropriate applicator is selected. The radiation therapists then attach the selected pair of applicators using the coordinates provided by the physician. Prior to commencing treatment, applicator coordinates used, final separation, and cable attachments are checked before treatment begins. The above procedure is then repeated for the next field. Depending on source strength, the treatment time is usually under 10 minutes per axis.
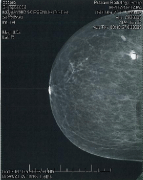
Figure 1: Craniocaudal pre-surgical mammogram.
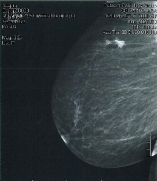
Figure 2: Medial lateral pre-surgical mammogram.
Compression required for a treatment mammogram is typically on the order of probably 20% less than what is required for a diagnostic study. The minimum breast separation is greater than 3 cm. When using the standard round or D applicators, the maximum separation needs to be less than 8 cm but can be up to 10 cm using the newer conical round Skin-Dose Optimized (SDO) applicators. This translates to a maximum of 14 Deca Newtons (DAN) on the compression scale, with the typical patient requiring about 10 DAN. Our experience has been that in most instances the first NIBB simulation may be sub optimal but gives the treating physician an idea whether treatment is feasible. The images obtained on the actual treatment day are usually much better and more conducive for treatment than the simulation films, as both the patient and radiation therapists know what to expect and mutually cooperate to get an optimal image (Figures 3 and 4).
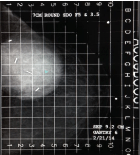
Figure 3: Day 1 first attempt.
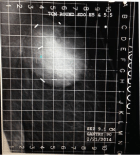
Figure 4: Second attempt.
Once the radiation oncologist identifies the tumor bed on the CC and ML mammogram, the next step in the work flow is to determine which applicator appropriately covers the tumor bed. We started by using regular C & D applicators which come in various sizes, the D shaped applicator generally being used for lesions close to the chest wall. Our current practice is to use the newer circular applicators with a central cone that enables the dose to be driven deeper plus allowing the maximum separation to in some instances to be increased to 10 cm. Physicians are urged to play close attention to the Skin Center Distance (SCD), (Figure 5).
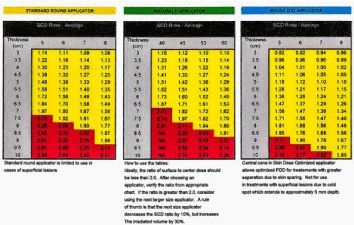
Figure 5: Comparison of SCD ratios between standard round and cone applicator series.
It is important to ensure that the ratio of the Surface to Center Dose does not exceed 2.0. To avoid entering the “Red Zone”, utilization of a larger size applicator is recommended. As has been depicted in the ML set up demonsrated below, a 7 cm applicator was utilized instead of the initially chosen 6cm applicator. Although the 6cm applicator provided adequate coverage, it would have resulted a “hot” treatment, since the best separation the patient could tolerate comfortably was 9.7cm (Figure 6).
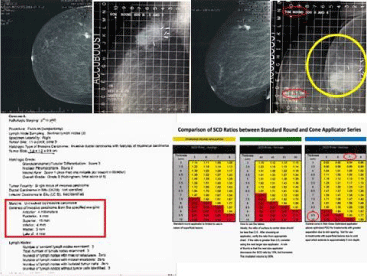
Figure 6: Comparison of SCD ratios.
In this patient, the clips are clustered posteriorly, raising concern about a potential posterior geographic miss. However, the actual site of the lesion on pre-surgical mammogram and clear posterior margin on pathology report allowed the physician to select the appropriate applicator and be assured that tumor bed was not being under covered. Additionally, dosimetric evaluations have shown that there is likely dose deposited approximately 5mm posterior to the edge of the applicator, allowing for an additional safety margin.
Results
Patient characteristics
All patients seen at our center with early stage breast cancer were offered primary surgeries or BCS followed by WBRT and NIBB. Of the 91 patients seen between 2011 and 2014, 7 patients elected to undergo modified radical mastectomy. 13 patients underwent BCS followed WBRT with either an electron beam or photon boost. 71 patients (78%) received WBRT followed by a boost using NIBB. Median age was 62 (range of 42-62). The vast majority had tumor size T2 or smaller and clinical stage II or lower. 20 patients (28%) had DCIS. 27 patients had breast bra size of D or greater. The data is summarized in Table 1.
PATIENT CHARACTRISTICS
Patients treated with NIBB, n
71
Age (mean)
62
T-Stage, n (%)
TIS
18 (25%)
T1a
11 (15%)
T1b
10 (14%)
T1c
18 (25%)
T2
10 (14%)
T3
4 (7%)
Node Status, n (%)
N0
20 (28%)
N1
12 (17%)
N2
2 (3%)
Overall Stage, n (%)
DCIS
20 (28%)
I
35 (49%)
II
14 (20%)
III
2 (3%)
Breast Cup Size, n (%)
A
1 (1%)
B
16 (23%)
C
27 (38%)
D
20 (28%)
DD
7 (10%)
Table 1: Patient characteristics.
Treatment summary
Of the 71 patients treated with BCS, all received WBRT to a total dose of 45Gy delivered in 18Gy fractions over 5 weeks. NIBB was usually delivered at the start of therapy to a total dose of 1600Gy using 2Gy fractions. Adjuvant chemotherapy was administered to 44 (62%) patients.
Skin toxicity
Skin toxicity was evaluated using Common Toxicity Criteria version 4.0. The majority of patients (83%) had Grade 0 acute reaction. Only 6% had Grade 1 toxicity and 4% had Grade 2 toxicity. No patients had Grade 3 or 4 toxicity. Late toxicity consisting of telangiectasia or fibrosis occurred in only 6 (9%) of the patients. Results are summarized in Table 2.
Acute Toxicity
Grade 0, n (%)
59 (83%)
Grade 1, n (%)
8 (11%)
Grade 2, n (%)
4 (6%)
Grade 3, n (%)
0
Grade 4, n (%)
0
Late Toxicity
Grade 0, n (%)
65 (91%)
Grade 1, n (%)
4 (6%)
Grade 2, n (%)
2 (3%)
Grade 3, n (%)
0
Grade 4, n (%)
0
Table 2: Skin toxicity was evaluated using Common Toxicity Criteria version 4.0.
Cosmesis
Cosmesis was assessed using the Harvard/RTOG scale. 90% of the patients had an excellent or good cosmetic result six months after completing therapy. Only 7% had a fair result and 3% had a poor result.
Discussion
Electron boosts when used can result in permanent undesirable telangiectatic changes. Figure 7 demonstrates a patient who received electron beam boost to her right breast and NIBB boost to her left breast, with identical whole breast radiotherapy. Clearly, NIBB provided a superior cosmetic outcome.
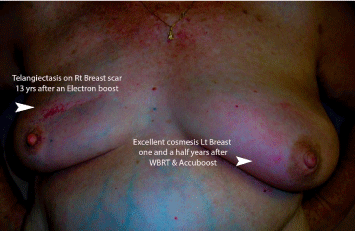
Figure 7: Electron boosts when used can result in permanent undesirable
telangiectatic changes.
Both photon and electron boosts can result in long term rib and chest wall tenderness. NIBB facilitates irradiation of the tumor bed with complete sparing of these structures. (Figure 8 and 9).
Figure 8: Both Photon and electron boosts can result in long term rib and
chest wall tenderness.
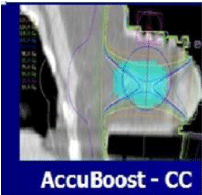
Figure 9: NIBB facilitates irradiation of the tumor bed with complete sparing
of ribs and chest wall.
Additionally, electron or photon 3DCRT or IMRT boosts (Figure 4, 5 and 6) commonly result in irradiation of these chest wall structures with the undesirable long term consequences that NIBB avoids as shown in the plans by each modality in Figure 10, 11 and 12.
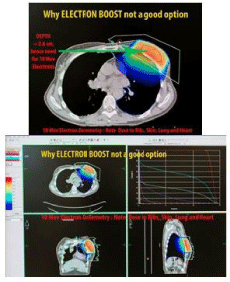
Figure 10: Electrons commonly result in irradiation of rib and chest wall
structures.
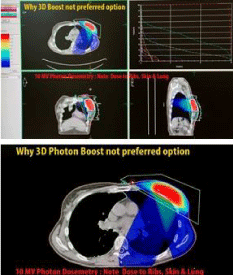
Figure 11: Photon 3DCRT commonly results in irradiation of rib and chest
wall structures.
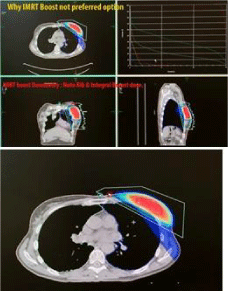
Figure 12: IMRT boosts commonly result in irradiation of rib and chest wall
structures.
NIBB followed by WBRT can be safely administered in a community setting for patients with early stage breast cancer. Our results are comparable with other reported studies. For acute skin toxicity, the series by Leonard et al. compared patients treated with Electron Beam (EB) boost versus NIBB [6]. NIBB had less acute toxicity than EB. A multi-center trial reported by Hamid et al. showed an acute skin toxicity of Grades 2 and greater of 18%, which was higher than our rate of 6% [5] summarized in Table 3.
Acute Skin Toxicity
Leonard, Electron Boost
Leonard, NIBB
Hamid, NIBB
Kuruvilla, NIBB
Grade 2, 3
52%
39%
18%
6%
Table 3: NIBB followed by WBRT can be safely administered in a community setting for patients with early stage breast cancer.
Cosmesis observed 6 months after treatment was evaluated using the Harvard & RTOG scale. Excellent or Good results were judged in about 90% of women in the Hamid et al. series, similar to our data. Fair or poor comprised about 10% of the results in both series (Table 4).
Cosmesis
Hamid, NIBB
Kuruvilla, NIBB
Excellent
67%
78%
Good
22%
12%
Fair
11%
7%
Poor
0
3%
Table 4: Cosmesis observed 6 months after treatment was evaluated using the Harvard & RTOG scale.
Conclusion
NIBB along with WBRT is an additional technique available for treating patients with early breast cancer following BCS. Several series have indicated that the treatment can be delivered safely and effectively with toxicities comparable to other modalities. Our experience confirms that this procedure can be implemented at community centers with similar outcomes.
References
- Clark RM, McCulloch PB, Levine MN, Lipa M, Wilkinson RH, Mahoney LJ, et al. Randomized clinical trial to access the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992; 84: 683-689.
- Fisher B, Anderson S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World J Surg. 1994; 18: 63-69.
- Barthelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of higher radiation dose on local control and survival in breast-conservation therapy of early breast cancer: 10 year results of the randomized boost versus no boost EORTC 22881-10882 trial. J ClinOncol. 2007; 25: 3259-3265.
- Benda RK, Yasada G, Seth A, Sheryl GA, Gabram MD, Russell W. Hinerman1et al. Breast boost: Are we missing the target? A dosimetric comparison of two boost techniques Cancer. 2003; 97: 905-909.
- Hamid S, Rocchio K, Arthur D, Vera R, Sha S, Jolly M, et al. A multiinstitutional study of feasibility, implementation, and early clinical results with noninvasive breast brachytherapy for tumor bed boost. Int J Radiat Oncol Biol Phys. 2012; 83: 1374-1380.
- Leonard KL, Hepel JT, Styczynski JR, Hiatt JR, Dipetrillo TA, Wazer DE. Breast boosts using noninvasive image-guided breast brachytherapy vs. external beam: a 2:1 matched-pair analysis. Clin Breast Cancer. 2013; 13: 455-459.