
Case Report
Austin J Radiat Oncol & Cancer. 2016; 2(1): 1017.
A Rare Presentation of Extra Pulmonary Small Cell Carcinoma at Tongue
Soni A¹*, Dhull AK¹, Verma R², Kaushal V¹ and Verma M³
1Department of Radiotherapy, University of Health and Sciences, India
2Department of Pathology, University of Health and Sciences, India
3Department of Biochemistry, University of Health and Sciences, India
*Corresponding author: Soni A, Department of Radiotherapy, University of Health and Sciences, 236G Model Town, Rohtak, India
Received: May 16, 2016; Accepted: June 01, 2016; Published: June 03, 2016
Abstract
Context: To report a case of extrapulmonary small cell carcinoma at a rare site of tongue with excellent prognosis with standard therapy.
Case: A 65-year-old previously healthy male presented initially with difficulty in swallowing and later, with a solitary neck lump and he was diagnosed as extrapulmonary small cell carcinoma of tongue. Unlike small cell carcinoma lung, extrapulmonary small cell carcinoma is uncommon and in base of tongue, it is extremely rare. Cisplatin (75 mg/m2) and Etoposide (100 mg/m2) were administered in combination at 3 weeks interval as neoadjuvant chemotherapy for 3 cycles, as well as concomitant chemotherapy for 3 cycles with external beam radiation in a dose of 64 Gy. The patient tolerated well the treatment.
Conclusion: The prognosis of extrapulmonary small cell carcinoma is felt to be extremely poor. Contrary to other reports, this case demonstrates that a good response with standard therapy is possible.
Keywords: Cisplatin; Poor; Tongue; Extrapulmonary; Radiation
Introduction
Extra Pulmonary Small Cell Carcinoma (EPSCC) was first described by Duguid and Kennedy in 1930, as a clinic pathological entity different from Small Cell Carcinoma Lung (SCLC) [1,2,3]. The small cell carcinoma usually develops from lung and approximate 2.5- 4% of it arises in extrapulmonary sites [4,5]. However, EPSCC is still often confused with metastatic SCLC [6]. Unlike SCLC, the natural history of EPSCC remains undiscovered and hence, optimal therapy determination is complicated [7]. The most commonly reported sites with EPSCC are aero-digestive tract including paranasal sinuses, nasal cavity, salivary glands, thyroid gland, larynx and trachea [8]. It has also been found rarely in ovaries, prostate gland, urinary bladder, cervix and breast [9]. In the head and neck region, oral cavity and oropharynx are the rarest sites for EPSCC. The EPSCC shares the similar histopathological features as of SCLC. The clinical course of EPSCC is more aggressive than SCLC and being more recurrent, it usually demonstrates a poorer prognosis [6,7]. The treatment of EPSCC patients has been very likely to the protocols used for treating SCLC. Due of its chemo sensitive nature, most of the EPSCC patients have been treated with Cis-platinum based chemotherapy [10]. As it is a systemic disease, localized treatment as a sole modality produces only limited survival; so, multimodality therapy is preferred even at early stage [10]. Present case is a primary extrapulmonary small cell carcinoma with nodal metastasis in ipsilateral upper cervical node without paraneoplastic feature and good response to treatment suggesting a different clinic pathophysiological behaviour than typical pulmonary small cell carcinoma.
Case Presentation
A 65 years old male with 30 pack year history of smoking presented to radiotherapy department with difficulty in swallowing for the past 3 months. Initially the patient reported difficulty in swallowing to solid food which later progressed to semisolid food and eventually patient got dependent upon liquid diet only. The patient also reported associated history of pain in head and neck region since the past 2 months, which was continuous, progressive and relieved only after medication. The patient also reported lump in left side of the upper neck which was gradually progressive in nature and was associated with pain. There was no history of chewing betel nuts or tobacco. The patient was non-alcoholic. There was no significant past history related to hypertension, diabetes mellitus, tuberculosis or any other chronic illness. No significant family history was reported. Oral cavity examination showed a large, proliferative growth at base of tongue associated with left sided upper deep cervical lymphadenopathy of neck level II, 3.5 × 2 cm firm, mobile palpable mass. Rest of the systemic examination was within normal limits.
Investigations
Routine blood biochemistry parameters were within normal limits. The fiber optic endoscopy showed a large, proliferative growth at left base of tongue, involving vallecula and left tonsil. On indirect laryngoscopy, both vocal cords were mobile and free of any growth. The fine needle aspiration cytology of the left sided neck node revealed small round cells with scant cytoplasm. As primary small cell carcinoma of the tongue is a rare entity, biopsy of the tongue was recommended for definitive diagnosis. The biopsy from growth base of tongue was consistent with small cell carcinoma (Figure 1a and 1b). The immunehistochemical staining was positive for CK, synaptophysin and chromogranin A, and was negative for LCA and p 63 (Figure 2a, 2b and 2c).
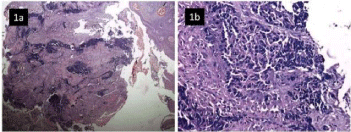
Figure 1: Overlying hyperplastic stratified squamous epithelium and
underlying soft tissue revealing infiltration by inflammatory exudate and
sheets of malignant small round cells (H&E 100x, 200x).
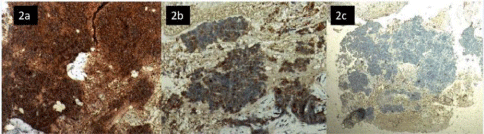
Figure 2: a) Synaptophysin strongly positive in malignant small round cell (IHC 400x). b) Cytokeratin focally positive in tumor cells (IHC 200x). c) LCA negative in
tumor cells (IHC 100 x).
Thus a diagnosis of small cell carcinoma of the tongue with metastasis to neck lymph node was confirmed. The pretreatment CECT scan revealed an ill defined heterogenous enhancing lesion with area of necrosis seen involving left hyoglossus muscle and left myelohyoid muscle along with involvement of left mesenteric space. Genioglossus muscle and left submandibular gland were also appeared to be involved. There is involvement of left vallecula and lesion is extending into subcutaneous fat on left side of neck (Figure 3). CECT scan revealed multiple enlarged necrotic lymph nodes in bilateral jugular group and posterior triangle with largest of size 3.7cm × 1.8cm (Figure 4). A large 3 cm sized lymph node was seen posterior to the left internal jugular vein. No additional lymph node was seen. On PET/CT scan, neither parenchymal lung lesion nor mediastinal lymphadenopathy was noted, suggesting non pulmonary origin of the tumor. Blood profiling, ECG and liver and kidney functions test carried out for any suspected paraneoplastic finding but all parameters were within normal limits suggesting no paraneoplastic association in this case.
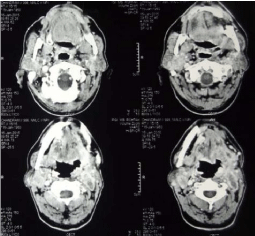
Figure 3: Pretreatment CECT scan revealing an ill defined heterogenous
enhancing lesion with area of necrosis involving left hyoglossus muscle,
left myelohyoid muscle, genioglossus muscle and left submandibular
gland. There is involvement of left vallecula and lesion is extending into
subcutaneous fat on left side of neck.
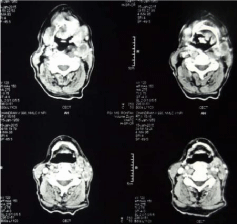
Figure 4: CECT scan revealing multiple enlarged necrotic lymph nodes in
bilateral jugular group and posterior triangle with largest of size 3.7cm x
1.8cm.
Differential diagnosis
Small cell carcinoma is poorly differentiated neuroendocrine carcinoma. It must be distinguished from basaloid squamous cell carcinoma, solid variant of adenoid cystic carcinoma, mucosal malignant melanoma and merkel cell carcinoma. Presence of squamous dysplasia is strongly supportive of the basaloid variant of squamous cell carcinoma. Immunohistochemistry is of limited value in its distinction from small cell carcinoma [11]. Malignant melanoma is strongly positive for HMB-45 and S-100 protein.
Treatment
The case was discussed in detail with a team comprising radiation oncologist, surgical oncologist, medical oncologist, as well as specialists in field of lung and head and neck cancer management, and it was decided to treat this case as extrapulmonary small cell carcinoma primarily arising from base of tongue. The tumor was staged as locally advanced (T4a N2a M0) stage disease, and the patient was planned to treat with neoadjuvant chemotherapy with injection cisplatin and etoposide intravenously, then followed by concomitant chemotherapy and radiation. As extrapulmonary small cell carcinoma is of aggressive nature, it was decided to treat him with Cisplatin (75 mg/m2) and Etoposide (100 mg/m2) every 3 weeks concomitant with external beam radiation therapy. The patient completed 3 cycles of neoadjuvant chemotherapy and subsequently received 2 cycles of concomitant chemotherapy and total 64 Gy by bilateral parallel opposed field on telecobalt machine.
Outcome and follow-up
During treatment patient developed severe grade 3 mucositis and required parenteral nutrition and symptomatic treatment. The follow up PET/CT scan after 6 months treatment shows complete resolution of the primary lesion at tongue as well as neck mass.
Discussion
Small cell carcinoma usually develops from the lung and represents ~20–25% of all bronchogenic carcinomas [12]. Only about 2.5-4% of small cell carcinoma arises in extrapulmonary sites as EPSCC [4,5]. EPSCC represents an overall incidence of 0.1%- 0.4% of all cancer [13]. However, EPSCC is still often confused with metastatic SCLC [6]. EPSCC found most commonly in aero-digestive tract including paranasal sinuses, nasal cavity, salivary glands, thyroid gland, larynx and trachea and rarely in ovaries, prostate gland, urinary bladder, cervix and breast [8,9]. For oropharyngeal carcinoma, the most common histopathology seen is squamous cell carcinoma, although other histologies like sarcoma, lymphoma etc are very rarely seen [14]. Only 0.2 to 1.6% of all tongue cancers are metastatic deposits [6,15]. In head and neck region, larynx is most common site followed by salivary glands, oral cavity and oropharynx are the rarest sites for EPSCC. EPSCC affects patients of middle age [12]. It is more common in male and in smokers [4,8]. Some of the previous studies have demonstrated that smoking is associated positively with EPSCC of particular sites particularly the head and neck or esophagus [7]. Present case also shows a tendency for this correlation.
SCLC is a very rapidly growing tumor with potential of distant metastasis even in early course of disease. It usually gets metastasized to bones, lymph nodes, adrenal gland, liver, oral cavity, tongue, gingival, parotid gland and brain [15]. EPSCC is a separate and very rare clinicopathological entity, but its clinical course is aggressive like SCLC, with early dissemination, and being recurrent in nature, it carries a poor prognosis [16]. The patient in this study revealed no respiratory symptoms and short duration history of primary tongue mass and neck mass. Aggressive nature of the disease is revealed by the neck lymphadenopathy at the time of the diagnosis.
The EPSCC shares the similar histopathological features as of SCLC [10]. The pathological biopsy demonstrated cells with hyper chromatic nuclei, scanty cytoplasm, inconspicuous nucleoli, and a fragment showing fibrocollagenous tissue revealing infiltration by malignant small round cells. The immunohistochemistry showed focal positivity for CK which is consistent with small cell carcinoma. The immunohistochemistry for melanoma, primary breast, colon, lung and lymphoma were negative. On immunohistochemistry, the tumor cells were positive for neuroendocrine markers such as synaptophysin and chromogranin A, as reported by Kim and Latif et al., [8,9,17]. The treatment of EPSCC patients has been very likely to the protocols used for treating SCLC. Multimodality therapy is preferred even at an early stage [8,9,18]. The patients are divided into 2 groups, limited or extensive disease. Limited Disease (LD) is defined as localized tumor with or without regional lymphadenopathy and is easily encompassed within a radiation field. Any type of extension beyond locoregional boundaries was defined as an Extensive Disease (ED) [2,8]. Multimodality therapy including chemotherapy in combination with radical surgery or radiotherapy is needed for patients with LD. The chemotherapeutic combinations used for treating EPSCC are similar to those used to treat SCLC [8]. The most common regimen used is the combination of etoposidecisplatinum or camptothecin- cisplatinum. The combination of cisplatin and etoposide is most commonly used regimen, with 69% response rate [12]. Median Overall Survival (OS) is 9.6 months for LD EPSCC disease and 9.2 months for ED EPSCC disease [12]. For EPSCC patients with ED, palliative therapy is given essentially. Cure is certainly possible in LD, and aggressive therapy is recommended. Complete resection is rarely achieved in EPSCC. In EPSCC patients with LD, chemoradiation (including platinum based chemotherapy) should be the primary form of management. RT should cover primary tumor and involved regional lymph nodes to a dose of 50 Gy at least of 2 Gy per fraction [19]. In the present case, the patient was treated with neoadjuvant Cisplatin and Etoposide chemotherapy followed by 3 weekly cisplatin based concomitant radiation therapy with excellent response to the treatment and patient is disease free after 6 months of treatment.
Conclusion
To conclude, oropharanx (Base of tongue) can be one of the rare site for extrapulmonary small cell carcinoma. EPSCC is treated on the same protocols as for SCLC but no clear recommendations are there because of paucity of cases and very few studies. Contrary to literature pertaining to extrapulmonary small cell carcinoma, good response with standard therapy is possible with meticulous treatment planning and multimodality interdisciplinary care.
Consent
Written informed consent was obtained from the patient for publication of this report.
References
- Duguid JB, Kennedy AM. Oat-cell tumors of mediastinal glands. J Pathol Bacteriol. 1930; 33: 93-99.
- Richardson RL, Weiland LH. Undifferentiated small cell carcinomas in extrapulmonary sites. Semin Oncol. 1982; 9: 484-496.
- Galanis E, Frytak S, Lloyd RV . Extrapulmonary small cell carcinoma. Cancer. 1997; 79: 1729-1736.
- Ledermann JA. Extrapulmonary small cell carcinoma. Postgrad Med J. 1992; 68: 79-81.
- Terashima T, Matsuzaki T, Kawada I, Nishida J, Tanaka Y, Morishita T, et al. Tongue metastasis as an initial presentation of a lung cancer. Intern Med. 2004; 43: 727-730.
- Remick SC, Hafez GR, Carbone PP. Extrapulmonary small-cell carcinoma. A review of the literature with emphasis on therapy and outcome. Medicine (Baltimore). 1987; 66: 457-471.
- van der Heijden HF, Heijdra YF. Extrapulmonary small cell carcinoma. South Med J. 2005; 98: 345-349.
- Kim KO, Lee HY, Chun SH, Shin SJ, Kim MK, Lee KH, Hyun MS. Clinical overview of extrapulmonary small cell carcinoma. J Korean Med Sci. 2006; 21: 833-837.
- Latif N, Rosa M, Samian L, Rana F. An unusual case of primary small cell neuroendocrine carcinoma of the breast. Breast J. 2010; 16: 647-651.
- Christodoulou C, Skarlos DV. Treatment of small cell lung cancer. Semin Respir Crit Care Med. 2005; 26: 333-341.
- Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol. 2002; 15: 264-278.
- Kim JH, Lee SH, Park J, Kim HY, Lee SI, Nam EM, et al. Extrapulmonary small-cell carcinoma: a single-institution experience. Jpn J Clin Oncol. 2004; 34: 250-254.
- Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, Alvi R. Extrapulmonary small cell cancer: a Canadian province's experience. Cancer. 2006; 107: 2262-2269.
- Licitra L, Bernier J, Grandi C, Merlano M, Bruzzi P, Lefebvre JL. Cancer of the oropharynx. Crit Rev Oncol Hematol. 2002; 41: 107-122.
- Yildiz O, Buyuktas D, Ekiz E, Selcukbiricik F, Papila I, Papila C. Facial nerve palsy: an unusual presenting feature of small cell lung cancer. Case Rep Oncol. 2011; 4: 35-38.
- Sengoz M, Abacioglu U, Salepci T, Eren F, Yumuk F, Turhal S. Extrapulmonary small cell carcinoma: multimodality treatment results. Tumori. 2003; 89: 274-277.
- Latif N, Imtiaz S, Wang B, Fauzia Rana and David Wolfson. Primary Small Cell Carcinoma of the Tongue. J Cancer Sci Ther. 2012; 5: 7.
- N Jonas, J Fagan, H Wu, et al. Neuroendocrine small cell carcinoma of the larynx. The Internet Journal of Otorhinolaryngology. 2007; 8: 1.
- Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010; 116: 888-895.