
Research Article
Austin J Radiat Oncol & Cancer. 2016; 2(1): 1018.
Comparison of Contemporary Treatment Options for Early Prostate Cancer: A Single Institution Series
Musunuru HB¹, Klotz L², Vesprini D¹, Morton G¹, Cheung P¹, Chung H¹, Sethukavalan P¹, Ghanem G¹, Zhang L¹, Mamedov A¹, Jethava V², D’Alimonte L¹, Deabreu A¹, Jain S³, Yamamoto T² and Loblaw DA1,4*
¹Odette Cancer Centre, Sunnybrook Health Sciences Centre, Canada
²Department of Surgical Urology, Sunnybrook Health Sciences Centre, Canada
3Centre for Cancer Research and Cell Biology, Queens University Belfast, Canada
4Department of Health Policy, Measurement and Evaluation, University of Toronto, Canada
*Corresponding author: Andrew Loblaw, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Department of Health Policy, Measurement and Evaluation, University of Toronto, Canada
Received: June 01, 2016; Accepted: June 08, 2016; Published: June 10, 2016
Abstract
Purpose: To evaluate outcomes of low-risk prostate cancer patients managed with competing treatments, in a single institution.
Methods: Patients with low-risk cancer (cT1-T2a and Gleason score 6 and PSA<10ng/ml) from 2006-2008 were included. Treatment details and worst late toxicities (Common Terminology Criteria for Adverse Events version 3.0) were retrieved through retrospective chart review. Biochemical relapse-free survival following primary (pbRFS) and salvage treatments (sbRFS), metastasis-free (MFS), cause-specific (CSS) and overall survival (OS) were also evaluated.
Results: In 582 patients, treatment options were active surveillance (AS, N=181), radical prostatectomy (RP, N=59), external beam radiation (EBRT, N=66; 76 Gy in 38 fractions), low-dose rate brachytherapy (LDR, N=192), stereotactic ablative radiotherapy (SABR, N=84; 35Gy in 5, weekly fractions). Median follow-up was 72.8 months. Six-year pbRFS and sbRFS were 94.0% and 95.8%, 84.1% and 98.3%, 92.1% and not applicable, 94.4% and not applicable, 95.8% and 98.7%; for AS, RP, EBRT, LDR and SABR, respectively. In multivariate analysis, RP had inferior pbRFS compared to EBRT, LDR or SABR (p-value <0.05) and a trend for AS (p-value 0.15). CSS, MFS and OS were similar. Toxicities were minimal in AS cohort. EBRT patients had higher rates of dysuria (19.7%), transurethral resection of prostate (6.1%) and hematochezia (7.6%). One patient each in EBRT, LDR and SABR cohorts had grade 4 toxicity. Toxicity data was not available for RP.
Conclusion: In primary setting all treatment modalities apart from RP had a 6-year pbRFS >90%, likely due to selection bias. Following salvage therapy, sbRFS was >95.0% in AS, RP and SABR cohorts.
Keywords: Prostate cancer; External beam radiotherapy; Stereotactic body radiotherapy; Brachytherapy; Active surveillance; Biochemical outcomes
Abbreviations
AS: Active Surveillance; RP: Radical Prostatectomy; LDR: Low- Dose Rate Brachytherapy; EBRT: External Beam Radiation; SABR: Stereotactic Ablative Radiotherapy; PSA: Prostate-Specific Antigen; TURP: Transurethral Resection of Prostate; IPSS: International Prostate Symptom Score; 3DCRT: Three-Dimensional Conformal Radiation; IMRT: Intensity Modulated Radiation Therapy; PTV: Planning Target Volume; pbRFS: Primary Biochemical Relapse- Free Survival; sbRFS: Salvage Biochemical Relapse-Free Survival; AUA: American Urology Association; ASTRO: American Society for Radiation Oncology; HDR: High-Dose Rate Brachytherapy; MFS: Metastasis-Free Survival; CSS: Cause-Specific Survival; OS: Overall Survival; GU: Genitourinary; GI: Gastrointestinal; CTCAE: Common Terminology Criteria for Adverse Events; APC: Argon Plasma Coagulation; CI: Confidence Intervals; SAS: Statistical Analysis Software; ADT: Androgen Deprivation Therapy; NCCN: National Comprehensive Cancer Network; EAU: European Association of Urology; ASCO: American Society of Clinical Oncology
Introduction
Management options for low-risk localized prostate cancer include Active Surveillance (AS), radical prostatectomy (RP), radical radiation including Low-Dose Rate Brachytherapy (LDR), External Beam Radiation (EBRT) and more recently SABR (stereotactic ablative radiotherapy) [1,2]. Designing randomized studies to compare these options has proven to be challenging due to predetermined patient’s choice of treatment, influenced by multiple factors [3]. Comparison of all available treatment options including contemporary radiotherapy modalities like SABR has not been performed on a single platform. Paucity of such data led to the inception of this study, comparing outcomes in low-risk prostate cancer patients, managed in a single high-volume academic institution.
Methods
This study was approved by Sunnybrook Health Sciences Centre, Toronto; Research Ethics Board (REB 066-2011).
Patients
Low-risk prostate cancer patients diagnosed on initial biopsy (reviewed by Uropathologist) and managed on institution specific protocols from January 2006 – December 2008 were selected based on retrospective chart review. Eligibility criteria consisted of clinical stage T1-T2a and Gleason sum score 6 and prostate-specific antigen (PSA) <10ng/ml. Management protocols were either AS, open RP, EBRT, LDR or SABR. Choice of treatment was based on baseline urinary symptoms, prostate volume, fitness for anesthesia and predominantly patient preference. Transurethral resection of prostate (TURP), prostate size >60cm3or pubic arch interference made patients ineligible for low-dose rate brachytherapy; International Prostate Symptom Score (IPSS)>19 or prostate size >90cm3 excluded them from SABR studies.
Protocols
AS was initiated in 1995 and data has been prospectively collected for over 1000 patients to date [4]. In short, patients on an AS pathway have 3 monthly PSAs, clinical examination and protocol biopsies every 3 years, following reconfirmation biopsy at year 1. Clinical progression, change in PSA kinetics with a PSA doubling time less than 3 years or pathological upgrading; constitute the triggers for active treatment.
Patients in the surgical cohort underwent standard open RP.
Prostate EBRT was delivered as three-dimensional conformal radiation (3DCRT) or intensity modulated RT (IMRT) with a median dose of 76Gy in 38 fractions. Planning target volume (PTV) margin for prostate was 10mm except posteriorly (7mm).
LDR brachytherapy patients had standard Iodine-125 interstitial implant with a minimal peripheral dose of 145Gy [5].
SABR patients were treated on a phase I/II prospective study (pHART3) [6]. Gantry-based SABR was delivered to a dose of 35Gy in 5, weekly fractions. A 4mm margin was added to the prostate for PTV. Treatment was delivered using step and shoot IMRT, gold seed fiducials were used for image guidance.
Study endpoints
Co-primary endpoints were biochemical relapse-free survival following primary (pbRFS) and local salvage therapies (sbRFS).
Patients on AS who did not receive treatment were censored as relapse-free at the time of bRFS analysis. Date of registration (i.e., first positive) biopsy was set as day zero for these patients.
In RP patients, date of surgery was day zero. Biochemical failure was defined as per American Urology Association (AUA)/American Society for Radiation Oncology (ASTRO) consensus (a confirmed PSA value > 0.2 ng/ml or one PSA > 0.4 ng/ml) [7]. Patients who had adjuvant radiotherapy were included in the primary RP cohort. Data about salvage radiotherapy was used to compute sbRFS. Biochemical failure following postoperative radiation was defined as per Phoenix criteria (nadir PSA following adjuvant radiation + 2.0ng/ml) [8], to facilitate fair comparison of modalities.
For EBRT and SABR patients, time zero was defined as the start of radiation. Phoenix definition [8] was used to identify biochemical failure following primary or salvage therapy. Salvage therapy could be either RP or focal high-dose rate (HDR) brachytherapy (Institutional phase I/II study, NCT01583920).
Secondary endpoints include Metastases-Free Survival (MFS), Cause-Specific Survival (CSS), Overall Survival (OS) and toxicities.
Electronic charts were reviewed to collect data about clinically significant bladder and bowel toxicities for patients managed on AS and radiotherapy protocols. Data about toxicity for study and nonstudy patients was collected using a standardized proforma at every clinic visit and documented in the chart. This data was retrospectively reviewed by a single physician to identify clinically significant worst toxicity at any point in the late follow-up period (>3 months following treatment), in order to minimize inter-observer bias and discrepancy associated with retrospective and prospective cohorts. For Genitourinary (GU) toxicities, significant dysuria needing more than one bladder medication was graded as grade 2 toxicity (Common Terminology Criteria for Adverse Events [CTCAE] version 3.0 [9]); late catheterization, late hematuria with clots needing catheter placement or admission, TURP, urethral stricture and fistula were reported separately.
For gastrointestinal domain, late GI bleed related to radiation needing any medical intervention in the form of steroid or mesalamine suppositories, 4% formalin therapy or argon plasma coagulation (APC) was reported as grade 2 toxicity. In addition, patients needing APC to control bleeding were reported separately as this is considered to be significant bleeding. GI stricture and fistula were recorded. Descriptive toxicity (where possible) rather than CTCAE grading was used to aid clarification.
Statistical analyses
Descriptive analysis was reported as median for continuous variables, and proportions for categorical variables. Primary bRFS was computed using Kaplan–Meier curve with 95% confidence intervals (CI). Patients who had biochemical control following salvage therapy were censored as disease-free at the time of sbRFS analysis. Information about patients with metastatic disease, deaths from prostate cancer and from all causes including prostate cancer, was used to compute MFS, CSS and OS, respectively.
As RP is considered to be a very well established treatment for prostate cancer, pair-wise comparison of primary bRFS between RP and each treatment (AS, LDR, SBRT, or EBRT) was conducted using log-rank test.
Univariate and multivariate analyses were performed to identify covariates predicting pbRFS after primary treatment. Age, age >65 versus =65 years, PSA at baseline (log scale), baseline PSA = 4.0ng/ml versus <4.0ng/ml, clinical stage T1 versus T2 and different treatment modalities (using RP as the reference treatment) were used as covariates in these analyses.
Fisher exact test was used to compare GU and GI toxicities. A second comparison was performed for only SABR and EBRT cohorts, given comparable patient selection criteria for these treatments.
All analyses were performed using Statistical Analysis Software (SAS version 9.2 for Windows).
Results
Five hundred and eighty-two patients were included in this study. One hundred and eighty-one patients were managed with AS, 59 patients underwent RP, 192 had LDR, 84 patients were treated with SABR and 66 patients had EBRT. Median follow-up for the entire cohort was 72.8 months (range 7.5-101.7months). Demographic details are summarized in Table 1. Biochemical and survival outcomes are described in Table 2; toxicities in Table 3.
AS
LDR
RP
SABR
EBRT
Fisher exact test
(N = 181)
(N = 192)
(N = 59)
(N = 84)
(N = 66)
p-value
Age (continuous)
N
181
192
59
84
66
<0.0001
Median (range) in years
66 (46-86)
62 (45-78)
68 (47-81)
67 (48-82)
68 (48-82)
Age (categories)
= 65
80 (44.2%)
121 (63.0%)
24 (40.7%)
34 (40.5%)
22 (33.3%)
<0.0001
> 65
101 (55.8%)
71 (36.9%)
35 (59.3%)
50 (59.5%)
44 (66.7%)
Clinical stage
T1
162 (89.5%)
160 (83.8%)
49 (83.0%)
78 (93.9%)
52 (78.8%)
0.02
T2
19 (10.5%)
31 (16.2%)
10 (16.9%)
5 (6.0%)
14 (21.2%)
Pre-treatment PSA (ng/ml)
< 4
77 (42.5%)
22 (11.5%)
12 (20.3%)
12 (14.5%)
6 (9.1%)
<0.0001
= 4
104 (57.5%)
170 (88.5%)
47 (79.7%)
71 (85.5%)
60 (90.9%)
Pre-treatment PSA
N
181
192
59
84
66
<0.0001
Median (range) ng/ml
4.71 (0.3-9.8)
5.68 (0-10)
5.00 (0.9-9.4)
5.73 (0.8-10)
6.30 (0.5-10)
Abbreviations: AS: Active Surveillance; LDR: Low Dose Rate Brachytherapy; RP: Radical Prostatectomy; SABR: Stereotactic Ablative Radiotherapy; EBRT: External Beam Radiotherapy; PSA: Prostate Specific Antigen.
Table 1: Basic demographics of patients in different treatment cohorts.
6-Year Survival Outcomes
AS
(N = 181)
LDR
(N = 192)
RP
(N = 59)
SABR
(N = 84)
EBRT
(N = 66)
Log-rank Test
p-value
Primary bRFS (pbRFS)
0.04
No. of biochemical failures
10
9
9
3
5
pbRFS
(95% CI)
94.00%
(90.5-97.7%)
94.40%
(90.6-98.3%)
84.10%
(74.3-95.2%)
95.80%
(91.3-100%)
92.10%
(85.6-99.0%)
Salvage bRFS (sbRFS)
0.59
No. of biochemical failures
7
9
3
1
5
sbRFS
(95% CI)
95.80%
(92.7-98.9%)
94.40%
(90.6-98.3%)
98.30%
(95.0-100%)
98.70%
(96.1-100%)
92.10%
(85.6-99.0%)
MFS
0.94
No. of patients with metastasis
2
2
0
1
1
MFS
(95% CI)
98.90%
(97.3-100%)
98.90%
(97.3-100%)
100%
98.70%
(96.1-100%)
98.50%
(95.6-100%)
CSS
0.46
No. of prostate cancer deaths
0
1
0
0
1
CSS
(95% CI)
100%
99.30%
(97.9-100%)
100%
100%
98.50%
(95.6-100%)
OS
0.72
No. of deaths
(any cause)
9
5
2
2
3
OS
(95% CI)
94.60%
(91.3-98.1%)
97.60%
(95.2-100%)
98.30%
(95.1-100%)
97.60%
(94.3-100%)
95.30%
(90.3-100%)
Abbreviations: CI: Confidence Interval; AS: Active Surveillance; LDR: Low Dose Rate Brachytherapy; RP: Radical Prostatectomy; SABR: Stereotactic Ablative Radiotherapy; EBRT: External Beam Radiotherapy; bRFS: Biochemical Relapse-Free Survival; MFS: Metastasis-Free Survival; CSS: Cause-Specific Survival; OS-Overall Survival.
Table 2: Survival outcomes across various treatment cohorts.
AS
N=181
LDR
N=192
SABR
N=84
EBRT
N=66
Fisher exact test
p-value
GU toxicity
Grade 2 late dysuria
5 (2.8%)
23 (12.0%)
10 (12.0%)
13 (19.7%)
0.0005
Late catheter
0 (0.0%)
*6 (3.1%)
*1 (1.2%)
*1 (1.5%)
0.67
= Grade 2 late hematuria
1 (0.6%)
*1 (0.5%)
*2 (2.4%)
*3 (4.5%)
0.24
TURP
0 (0.0%)
1 (0.5%)
2 (2.4%)
4 (6.1%)
0.004
Stricture
5 (2.8%)
3 (1.6%)
0 (0.0%)
1 (1.5%)
0.50
Grade 4
0 (0.0%)
0 (0.0%)
0 (0.0%)
1 (1.5%)
NA
GI toxicity
Grade 2 rectal bleed
2 (1.1%)
7 (3.7%)
4 (4.8%)
5 (7.6%)
0.10
APC for GI bleed
2 (1.1%)
3 (1.6%)
1 (1.2%)
5 (7.6%)
0.07
GI Stricture
0 (0.0%)
0 (0.0%)
0 (0.0%)
1 (1.5%)
NA
Grade 4
0 (0.0%)
1 (0.5%)
1 (1.19%)
0 (0.0%)
0.51
Abbreviations: AS: Active Surveillance; LDR: Low Dose Rate Brachytherapy; SABR: Stereotactic Ablative Radiotherapy; EBRT: External Beam Radiotherapy; GU: Genitourinary (Bladder); GI: Gastrointestinal (Bowel); TURP: Transurethral Resection of prostate; APC; Argon Plasma Coagulation; NA: Not Available.
*Patients who experienced late hematuria and subsequently needed catheterization were included separately under both categories.
Table 3: GU and GI toxicities in four different treatment groups.
Predictive factors
Coefficient
Standard error
p-value
Hazard Ratio
95% CI
of Hazard Ratio
PSA at baseline (log scale)
2.28119
0.76611
0.002
9.78
2.18
43.93
Stage T2 vs. T1
0.98114
0.43021
0.02
2.66
1.14
6.19
Treatment Groups
0.015
AS vs. RP
-0.6641
0.46191
0.15
0.51
0.2
1.27
LDR vs. RP
-1.47867
0.4766
0.001
0.22
0.09
0.58
EBRT vs. RP
-1.20732
0.57093
0.034
0.29
0.09
0.91
SABR vs. RP
-1.6127
0.67232
0.016
0.19
0.05
0.74
Abbreviations: bRFS: Biochemical Relapse-Free Survival; PSA: Prostate-Specific Antigen; CI: Confidence Interval; AS: Active Surveillance; LDR: Low Dose Rate Brachytherapy; SABR: Stereotactic Ablative Radiotherapy; EBRT: External Beam Radiotherapy; Vs: versus.
Table 4: Covariates predicting primary bRFS following multivariate analysis.
Biochemical outcome
Active surveillance (AS): Median follow-up was 70 months (range 36.6-92.5 months). Forty-two patients (23.2%) on AS protocol underwent radical treatment. Treatment details were available for 39 patients. Median time to treatment was 66 months (range 6.5-92.4 months). Reclassification on subsequent biopsies was the indication for intervention in 51.2% of the treated cohort. 71.7% received radical radiation in the form of LDR, EBRT or SABR. Eleven patients received neo-adjuvant/adjuvant androgen deprivation (ADT) with radiation therapy.
Ten patients had biochemical failure, resulting in a 6-year pbRFS of 94.0% (95% CI 90.5% - 97.7%). Four patients had salvage therapy in the form of radiation following radical prostatectomy. After excluding patients who remained in biochemical control following salvage therapy, seven had biochemical failure, resulting in a 6-year sbRFS of 95.8% (95% CI 92.7% - 98.9%). Two patients developed metastatic disease following treatment.
Radical prostatectomy: Median follow-up was 75.4 months (range 9.6-100 months). Six patients (8.6%) had pT3a or higher disease, 35 patients (60.3%) had Gleason 7 disease and 20.3% had margin positive disease on postoperative histology. Majority of the cohort (88%) did not have lymphadenectomy due to their low-risk status. Nine patients developed biochemical failure; resulting in a 6-year pbRFS of 84.1% (95% CI 74.3% - 95.2%). Seven patients received salvage radiation; resulting in a 6-year sbRFS of 98.3% (95% CI 95.0% - 100%).
External beam radiation (EBRT): Median follow-up for EBRT was 80 months (range 7.5-99.2months). 83% received 76 Gy in 38 fractions, 5 fractions per week, either as 3DCRT or IMRT. Six patients received up to 6 months of ADT for cytoreduction. Five patients had biochemical failure following EBRT; resulting in a 6-year pbRFS of 92.1% (95% CI 85.6% - 99.0%). Salvage radical therapy was not used in this cohort. One patient developed metastatic disease and later died of disease.
LDR brachytherapy (LDR): Median follow-up was 73 months (range 25.0 -101.7months). Eight patients received neo-adjuvant ADT for cytoreduction.
Nine patients had biochemical failure, resulting in a 6 year-pbRFS of 94.4% (95% CI 90.6% - 98.3%). Salvage radical therapy was not used for any of these patients. Two patients developed metastatic disease and one patient died of it.
Stereotactic ablative radiotherapy (SABR): Median follow-up was 72 months (range 11.8-91.0months). One patient received neoadjuvant ADT for cytoreduction. Three patients had biochemical failure, resulting in a 6-year pbRFS of 95.8% (95% CI 91.3% - 100%). Two patients underwent salvage focal HDR brachytherapy; leading to a 6-year sbRFS of 98.7% (95% CI 96.1% - 100%). One patient developed metastatic disease and is being managed on ADT.
Comparison of survival outcomes: Using log-rank test, there were statistically significant differences in pbRFS between RP and AS (p=0.035; Hazard Ratio (HR) =0.39; Figure 1a), RP and LDR (p=0.005; HR=0.29; Figure 1b), RP and SABR (p=0.016; HR=0.23; Figure 1c) and a trend between RP and EBRT (p=0.151; HR=0.46; figure 1d), with RP resulting in inferior pbRFS. There was no statistically significant difference between RP and AS or SABR for sbRFS.
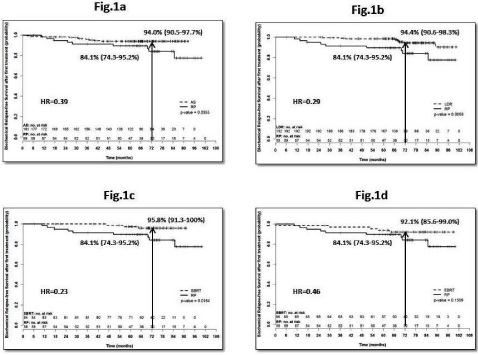
Figure 1: a) pbRFS comparing AS with RP, b) pbRFS comparing LDR with RP, c) pbRFS comparing SABR/SBRT with RP, d) pbRFS comparing EBRT with RP.
Abbreviations: bRFS: Biochemical Relapse-Free Survival; pbRFS: Biochemical Relapse-Free Survival following primary treatment; CI: Confidence Interval; AS:
Active surveillance; LDR-Low Dose Rate Brachytherapy; SABR: Stereotactic Ablative Radiotherapy; SBRT: Stereotactic Body Radiotherapy; EBRT: External
beam radiotherapy; RP: Radical Prostatectomy; HR: Hazard Ratio.
In univariate analysis, higher baseline PSA value (log scale), clinical stage T2 and specific treatment groups had lower bRFS following primary treatment. LDR (p=0.008) and SABR (p=0.025) had statistically significant superior pbRFS and there was a trend for AS (p=0.05) and EBRT (p=0.15); when individual treatment groups were compared with RP.
In multivariate analysis for pbRFS; baseline PSA value, stage T2, LDR vs. RP, SABR vs. RP and EBRT vs. RP were significant (Table 4). There were no statistically significant differences between MFS, CSS or OS between these five different modalities.
Toxicity
AS
One patient (0.6%) had significant late hematuria (grade 2) and 5 patients (2.8%) developed urethral stricture, following postoperative radiation. For patients treated with EBRT or SABR, 2 patients (1.1%) developed late grade 2 rectal bleed requiring APC.
RP
Toxicity details were not available for RP patients.
EBRT
Three patients (4.5%) had gross hematuria with clots in the late period. One (1.5%) of them required temporary catheterization for 5 months and the second patient (1.5%) needed cysto-prostatectomy (grade 4) to control bleeding. Four patients (6.1%) ended up having TURP and one patient (1.5%) developed urethral stricture.
Five patients (7.6%) developed grade 2 late radiation related rectal bleed needing (all requiring APC) and one patient (1.5%) developed ano-rectal stricture.
LDR
Six patients (3.1%) needed catheter in the late period with three patients (1.6%) requiring it permanently. One patient (0.5%) needed TURP and three patients (1.6%) developed urethral stricture.
Seven patients (3.7%) developed late grade 2 radiation related rectal bleeding; three (1.6%) of them needed APC. One patient (0.5%) developed recto-urethral fistula (Grade 4) requiring colostomy.
SABR
Two patients (2.4%) developed late =grade 2 hematuria, one following transurethral resection of bladder tumor and one due to benign prostatic hypertrophy; one (1.2%) of them required admission for continuous bladder irrigation. Two patients (2.4%) required TURP.
Four patients (4.8%) developed late grade 2 radiation related rectal bleed, one (1.2%) of whom required APC. One patient (1.2%) developed recto-cutaneous fistula (grade 4) but was managed conservatively.
Comparison of toxicity
EBRT patients had higher rates of late grade 2 or higher dysuria (19.7%, needing alpha blockers in combination with anticholinergics or antibiotics; p<0.001), TURP (6.1%; p=0.001) and late grade 2 radiation related rectal bleed needing medical interventions (7.6%; p=0.04) when compared to AS, LDR and SABR patients. EBRT group had higher number of patients needing APC for controlling radiation related rectal bleed (7.6%; p=0.03, Table 3). AS patients had minimal toxicity.
There were no statistically significant differences between EBRT and SABR, with respect to GU or GI toxicities.
Discussion
National Comprehensive Cancer Network (NCCN) and European Association of Urology (EAU) [10] have recognized Active Surveillance (AS), radical prostatectomy, external beam radiation and low-dose rate brachytherapy as treatment options for low-risk prostate cancer. ASTRO has also endorsed 5-fraction SABR as a standard treatment option for low-risk and intermediate-risk prostate cancer patients [2].
Having more than one effective treatment option poses a dilemma for both clinicians and patients. Information about efficacy following different treatment modalities, resultant toxicities and more importantly, quality of life changes might be valuable in this decision making process [11]. Given the scarcity of randomized studies comparing all available treatment options, well-conducted single institution studies might be an alternative solution to fill this existing void.
The current study is one of the largest single institution series comparing all available treatment options for localized prostate cancer. Results from this study demonstrate that the 6-year biochemical relapse-free survival was greater than 90.0% for all treatment modalities apart from RP (84.1%). This is likely due to the lower PSA threshold used to compute biochemical recurrence after RP compared to the non-surgical definitions although brachytherapy appears to have the same bRFS whether Phoenix, ASTRO or surgical PSA definitions are used [12,13]. An alternative reason could be selection bias, given that this is not a randomized study. Active surveillance, LDR and SABR had equivalent biochemical outcomes (=94.0%) following primary therapy. Volume of prostate receiving 100% of the prescribed dose (V100) was not significantly different between biochemical control and failure patients in the LDR cohort, attesting to the implant quality, although power was low to detect potential differences.
Utilization of salvage therapies varied amongst different cohorts resulting in variable salvage bRFS. 2.2%, 2.3% and 11.9% underwent radical salvage therapies in AS, SABR and RP cohorts, respectively; resulting in a 6 year-sbRFS higher than 95.0%. We believe that by allowing patients who had salvage postoperative RT to fail according to Phoenix definition, a fairer biochemical comparison between primary RP and RT could be done.
Among the non-surgical options, patients managed on AS protocol had the least GU/GI toxicities. Patients who underwent standard EBRT had higher GU/GI toxicities. This could be due to inherent patient selection bias or the predominant use of non-IMRT radiation techniques. Despite similar patient selection criteria and higher EQD2 (86.5Gy, α/β 1.4Gy [14]), fewer patients in SABR group experienced toxicities when compared to standard EBRT (difference not statistically significant, likely due to few events). One patient in each cohort (EBRT, SABR and LDR) developed grade 4 toxicity. Detailed information about toxicity was not available for RP patients.
If we were to evaluate different treatment options using efficacy and toxicity results from this study, AS might be the optimal solution for managing low-risk patients. Recent Cancer Care Ontario guidelines [15] (endorsed by ASCO [16]) recommended active surveillance as the preferred management strategy for lowrisk patients. Two randomized control trials comparing radical prostatectomy with monitoring used watchful waiting rather than active surveillance protocols [17,18]. Various single institution series, including the recently updated Sunnybrook experience have demonstrated the efficacy of AS in low-risk prostate cancer [4,19]. This is being compared against other treatment options in a large phase 3 UK ProtecT study [20].
In a Canadian cost comparison study, the mean cost of prostate cancer management over the first year and 5 years of follow-up was estimated at Cdn$6200 (95%CI $6083–$6317) per patient for AS and Cdn$13,735 (95% CI $13,615–$13,855) per patient with immediate treatment; resulting in an estimated economic benefit of $96.1 million for each annual cohort of incident prostate cancer [21]. Similar trends were observed within the US and Swedish studies [22-25]. Around a quarter of patients (23.2%) on the AS protocol received radical treatment in this study. The next predicament would be choosing between surgical and radiotherapy options upon progression on AS protocol. On the basis of this study, LDR brachytherapy and 5-fraction SABR have similar efficacy and not significantly different toxicity results. There are no studies comparing efficacy and quality of life outcomes for LDR brachytherapy and SABR. Reported outcomes in this study are comparable to large single institutional and multiinstitutional studies for LDR or SABR [26,27]. On the other hand, dose-escalated EBRT (76Gy in 38 fractions) did not fare well in the comparison of biochemical outcome or toxicity. This could be due to inherent selection bias or treatment effect. Radical prostatectomy has been compared with LDR or EBRT in randomized and nonrandomized studies [28,29], reporting contradictory outcomes. In our current study, radical prostatectomy resulted in slightly inferior bRFS, which could be due to selection bias. To our knowledge, this is the first study to report outcomes of five different management options in low-risk prostate cancer. Inclusion of active surveillance cohort, dose-escalated EBRT and novel radiation techniques like SABR and salvage therapies are unique for this study. Attention to detail about biochemical outcomes and toxicities in the context of median followup longer than 5 years, add strength to this study. Limitations of this study include its retrospective nature, median follow-up less than 10 years, different proportion of patients undergoing various treatments, missing toxicity data for RP group and lack of quality of life/costutility data for the entire cohort.
This study also highlights gaps in our existing knowledge about comparative quality of life [11] and cost effectiveness of the various treatment modalities. These outcomes would help clinicians and patients make conscientious decisions about treatment and should therefore be incorporated in future studies.
Conclusion
This comparative study demonstrates that active surveillance, LDR brachytherapy, 5-fraction SABR and dose-escalated external beam radiation have comparable efficacy in the primary setting. If salvage therapies were incorporated, then surgical and non-surgical treatment options have yielded equivalent biochemical outcomes.
Acknowledgement
A version of this work was presented at the 2014 Canadian Association of Radiation Oncology Annual Scientific Meeting in St. John’s, NL and 2015 GU Symposium in Orlando, FL.
References
- Keyes M, Crook J, Morton G, Vigneault E, Usmani N, Morris WJ. Treatment options for localized prostate cancer. Can Fam Physician. 2013; 59: 1269- 1274.
- ASTRO. Stereotactic Body Radiation Therapy (SBRT). ASTRO, Apr 17; 2013.
- Eccles BK, Cross W, Rosario DJ, Doble A, Parker C, Logue J, et al. SABRE 1 (Surgery Against Brachytherapy - a Randomised Evaluation): feasibility randomised controlled trial (RCT) of brachytherapy vs radical prostatectomy in low-intermediate risk clinically localised prostate cancer. BJU Int. 2013; 112: 330-337.
- Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Longterm follow-up of a large active surveillance cohort of patients with prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015; 33: 272-277.
- Keyes M, Crook J, Morris WJ, Morton G, Pickles T, Usmani N, Vigneault E. Canadian prostate brachytherapy in 2012. Can Urol Assoc J. 2013; 7: 51-58.
- Loblaw A, Cheung P, D’Alimonte L, Deabreu A, Mamedov A, Zhang L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: Toxicity, biochemical, and pathological outcomes. Radiotherapy and Oncology. 2013; 107: 153-158.
- Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013; 190: 441-449.
- Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006; 65: 965-974.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13: 176-181.
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011; 59: 61-71.
- Chen RC, Chang P, Vetter RJ, Lukka H, Stokes WA, Sanda MG, et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. Journal of the National Cancer Institute. 2014; 106.
- Morris WJ, Tyldesley S, Rodda S, R H, H P, McKenzie M, et al. OC-0845 LDR brachytherapy is superior to 78 Gy of EBRT for unfavourable risk prostate cancer: the results of a randomized trial. Radiat Oncol. 2015; 8140: 40481- 40485.
- Crook J. Long-term oncologic outcomes of radical prostatectomy compared with brachytherapy-based approaches for intermediate- and high-risk prostate cancer. Brachytherapy. 2015; 14: 142-147.
- Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9- 2.2) Gy. Int J Radiat Oncol Biol Phys. 2012; 82: e17-24.
- Morash C, Tey R, Agbassi C, Klotz L, McGowan T, Srigley J, et al. Active surveillance for the management of localized prostate cancer: Guideline recommendations. 2015; 9: 171-178.
- Chen RC, Rumble RB, Loblaw DA, Finelli A, Ehdaie B, Cooperberg MR, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. American Society of Clinical Oncology. 2016; 65: 7759.
- Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. The New England journal of medicine. 2014; 370: 932-942.
- Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England journal of medicine. 2012; 367: 203-213.
- Klotz L. Active Surveillance for Prostate Cancer: Debate over the Application, Not the Concept. Eur Urol. 2015; 67: 1006-1008.
- Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. The Lancet Oncology. 2014; 15: 1109-1118.
- Dragomir A, Cury FL, Aprikian AG. Active surveillance for low-risk prostate cancer compared with immediate treatment: a Canadian cost comparison. CMAJ Open. 2014; 2: E60-68.
- Eldefrawy A, Katkoori D, Abramowitz M, Soloway MS, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol. 2013; 31: 576-580.
- Corcoran AT, Peele PB, Benoit RM. Cost comparison between watchful waiting with active surveillance and active treatment of clinically localized prostate cancer. Urology. 2010; 76: 703-707.
- Wilson LS, Tesoro R, Elkin EP, Sadetsky N, Broering JM, Latini DM, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer. 2007; 109: 518-527.
- Andersson SO, Andren O, Lyth J, Stark JR, Henriksson M, Adami HO, et al. Managing localized prostate cancer by radical prostatectomy or watchful waiting: Cost analysis of a randomized trial (SPCG-4). Scandinavian journal of urology and nephrology. 2011; 45: 177-183.
- Morton GC, Hoskin PJ . Brachytherapy: current status and future strategies -- can high dose rate replace low dose rate and external beam radiotherapy? Clin Oncol (R Coll Radiol). 2013; 25: 474-482.
- King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiotherapy and oncology. 2013; 109: 217-221.
- Peinemann F, Grouven U, Bartel C, Sauerland S, Borchers H, Pinkawa M, et al. Permanent interstitial low-dose-rate brachytherapy for patients with localised prostate cancer: a systematic review of randomised and nonrandomised controlled clinical trials. Eur Urol. 2011; 60: 881-893.
- Sun F, Oyesanmi O, Fontanarosa J, et al. Therapies for Clinically Localized Prostate Cancer: Update of a 2008 Systematic Review. Comparative Effectiveness Review. 2014.