
Research Article
Austin J Radiat Oncol & Cancer. 2016; 2(2): 1021.
3-D Conformal Volumetric Alignment of Savi® Applicator Using Cone Beam Imaging Technique
Bhatnagar R¹*, James R¹, Simmons D¹, Berlin D², Brinkman J², Paryani S³, Paryani N³, Bhatnagar N4 and Kuruvilla A3
¹21st Century Oncology-Orange Park Cancer Center, USA
²North Florida Surgeons, Orange Park, Florida, USA
³First Radiation &Oncology Group, Jacksonville, Florida, USA
4University of Tennessee College of Medicine, Memphis, Tennessee, USA
*Corresponding author: Ravi Bhatnagar, 21st Century Oncology-Orange Park Cancer Center, 2161 Kingsley, Avenue, Orange Park, FL 32073, USA
Received: August 18, 2016; Accepted: September 21, 2016; Published: September 23, 2016
Abstract
We report a novel technique to confirm volumetric alignment of the SAVI® (Strut-Adjusted Volume Implant) applicator by utilizing fiducial markers embedded within the struts of the applicator. The technique compares Treatment Planning Cone Beam Images with daily, pre-treatment Cone Beam CT (CBCT) images obtained using the imaging option on a linear accelerator. The accelerator vault is also used for delivering High Dose Rate radiation treatment using Ir-192 source in a Nucletron after-loader immediately following matching of CBCT images. This allows for rapid verification of the correct position of SAVI Catheters without having to move patient when delivering HDR therapy.
Keywords: Cone beam CT, 3-D Conformal volumetric alignment, Savi®
Introduction
The SAVI technique for APBI (Accelerate Partial Breast Irradiation) offers some clinical and dosimetric advantages [1] over other devices currently on the market. The SAVI applicator consists of one (1) central catheter surrounded by as many as ten (10) collapsible peripheral catheters listed in Table 1. The fully expanded applicator is ellipsoid in shape and treatment volumes ranging from 20cc to 60cc can be treated effectively by selecting appropriate SAVI device. The catheter material is a fluoropolymer, which is biocompatible, highly inert, and lubricated making it resistant to adherence to tissue.
Design
One Central Catheter and six, eight or ten Peripheral Catheters
Dimensions
Lumpectomy Cavity
Size
Diameter (cm)
Long Axis (cm)
Volume (cm3)
6-1(mini)
2.4
5.0
20
6-1
3.0
6.1
30
8-1
4.0
6.7
40
10-1
5.0
7.5
60
x-Ray Markers on #2, #4 and #6 Peripheral Catheters
Table 1: Strut Adjusted Volume Implant (SAVI) Applicators.
Dose
(5% vol)
cGY/ fraction
Max. Dose
(<0.3% Vol)
cGY/ fraction
Max. Dose
(<0.3% Vol)
(cGy)
Total Dose
(cGy)
Original Plan
(2 fractions)
65
102
204
980.8
Revised Plan
(8 fractions)
54
97.1
776.8
Table 2: Dose to Heart.
The main dosimetry advantage of SAVI applicator is that isodose lines can be sculpted to conform to the treatment volume since a large number of catheters are available for treatment planning. In addition, the device can also be used when the separation between the lumpectomy cavity and the skin is as small as 2mm since skin-sparing can be achieved by differential loading of catheters. Such skin-sparing results in better cosmetic outcome and patient satisfaction [2]. In addition, several studies have been conducted reporting rates of infection, fat necrosis and seromas in patients who have undergone intra-cavitary balloon brachytherapy [3,4]. The design of the SAVI device allows for better drainage of seromas since there is no balloon obstructing its flow. The proposed technique of pre-treatment CBCT imaging also enables a physician to evaluate any fluid build-up in the lumpectomy cavity on a daily basis.
The collapsed applicator assembly is inserted through a small incision in the breast and positioned inside the lumpectomy volume. The choice of the applicator size is based on the lumpectomy volume. The applicator is fully expanded using a locking key that is inserted in the central catheter and turned clock-wise to expand and counter clock-wise to retract. The central stem of the SAVI device has a dial on the outside that is used to mark its location on patient’s skin, Figure 1. The device is not sutured to the skin and thus it may be subject to movement or rotation during the course of the radiation treatment. The length of central stem protruding outside of the skin shown in Figure 2 is also recorded on the day of CT Simulation to compare on following treatment days. The current recommended procedure is to use external skin marks corresponding to the dial position and orthogonal X-Ray images to verify the position of the device inside the cavity prior to each radiation treatment.
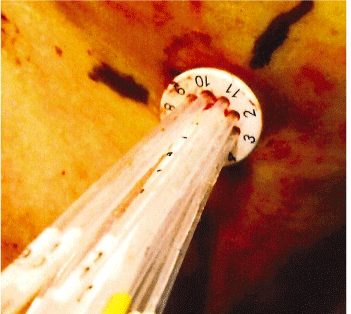
Figure 1: SAVI Position Indicator Dial.
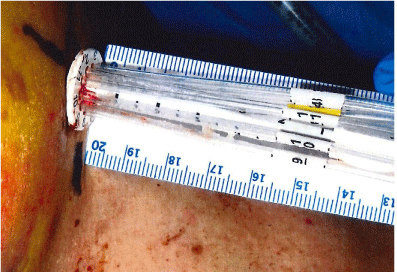
Figure 2: Length of Stem Out-side Patient’s Skin.
The objective of this study was to develop an alternate technique to the widely used and vendor recommended technique of orthogonal x-Ray images (kilo-voltage) or a CT-Scout using diagnostic imaging techniques.
Materials and Methods
X-Ray markers implanted within the SAVI device are easily visualized in kV images. As a free-standing cancer center, we did not have access to a portable C-Arm or an in-house CT scanner to image the patient in order to validate the integrity of the SAVI applicator before each treatment. However, we had access to a Siemens MD2 linear accelerator with Mega-Voltage imaging capabilities for Orthogonal or Cone Beam Images. The same accelerator vault was also used for routine HDR treatments.
Patients were evaluated by radiation oncologist and surgical oncologist to determine if she was a suitable candidate for SAVI procedure prior to lumpectomy procedure. Tissue samples were sent to a pathologist to confirm diagnosis and establish clear margins around the cavity. A dummy balloon was placed in the patient to prevent collapse of the lumpectomy cavity and to determine the volume of the cavity to choose appropriate size SAVI applicator. The balloon is left situated in the cavity during verification of the pathology (clear margins) and then replaced with appropriate size SAVI Applicator. The SAVI applicator is allowed to “set” in position for at least fortyeight hours as to limit the possibility of it rotating between the time of initial scan, and the time of treatment. If the scan is obtained prior to this time, the treatment plan would then potentially be generated too soon, and based upon the position of the catheters and fiducial markers which could subsequently change, thereby rendering the generated plan inaccurate and essentially useless. The development of small adhesions along the struts of the catheter is paramount to reproducibility. However, it is very important to complete the entire process, from implanting SAVI device in the cavity to the completion of radiation treatments in as short a time as possible, in order to minimize potential adhesion of catheters or stem to the tissue. Such tissue adhesion may increase patient’s discomfort when the applicator is retracted and removed from the lumpectomy cavity at the end of the treatment.
The patient was then brought to the radiation therapy clinic and positioned in a supine position with her arms above her head similar to simulation protocol for whole-breast external beam radiation therapy. A Vac-Loc was built to support her upper body and shoulders. A treatment planning CT (2mm slice thickness) was taken which was used for developing a brachytherapy plan using Oncentra Treatment Planning System as well as reference orthogonal and cone-beam CT images using CMS XiO treatment planning system. Two (2) mm CT slices were used to scan the treatment volume to ensure that small x-Ray markers embedded within the SAVI device appeared on at least one CT slice. These x-Ray markers were contoured as structures in XiO on corresponding CT slices and transferred to treatment console where each could be visualized independently. These orthogonal and cone beam images were used as reference images for daily comparison before treatment delivery.
Results
Figures 3 and 4 show AP and Lateral set-up images of SAVI applicator in a patient generated by CMS XiO treatment planning system.
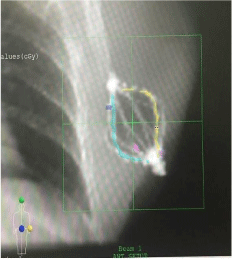
Figure 3: AP Set-up Image.
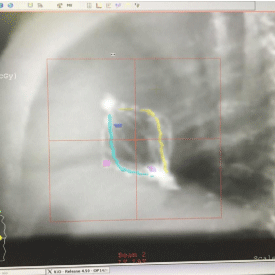
Figure 4: Lateral Set-up Image.
Figures 5 and 6 show Scout Films using Cone Beam Imaging taken on Siemens machine which were used to match fiducial markers to confirm alignment of SAVI applicator. Figure 7 shows 3-D Match of fiducial markers in Transverse, Sagittal and Frontal views using a kV Cone Beam image taken on a Varian Linear accelerator. We have now used the kV CBCT technique on fifteen SAVI patients in the past nine months and the whole alignment process can be completed in a relatively short time.
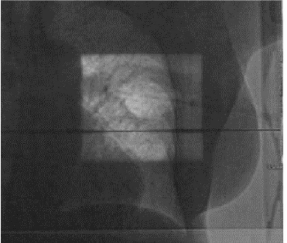
Figure 5: Scout Films Using Cone Beam Imaging from Siemens MD2.
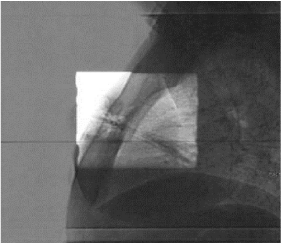
Figure 6: Scout Films Using Cone Beam Imaging from Siemens MD2.
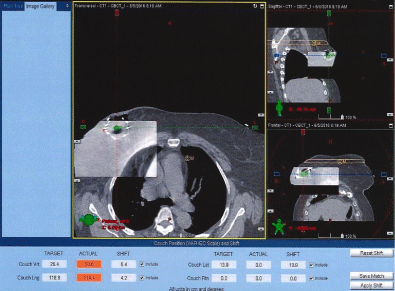
Figure 7: 3-D Match of Fiducial Markers on Varian Linac.
Figures 8 and 9 show two examples of SAVI treatment plans developed where skin-sparing was achieved using differential loading and another where the patient had large amount of fluid build-up in the lumpectomy cavity.
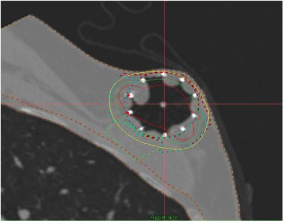
Figure 8: SAVI Applicator used for Skin-Sparing Case.
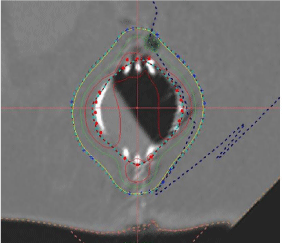
Figure 9: Cone Beam CT Image showing amount of fluid in the cavity.
We have also treated a patient in which a SAVI (8+1) device was placed in the lower left, inner quadrant of the left breast as shown in Figure 10.
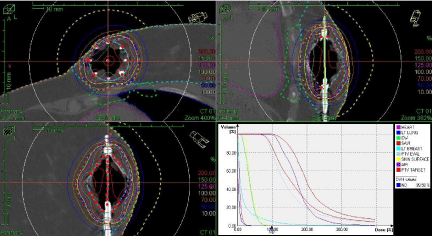
Figure 10: Minimizing Dose to Heart.
Discussion
The Cone Beam CT Images obtained on our accelerators were acceptable in allowing us to confirm SAVI catheter position for Breast HDR. There is good skin sparing obtained using the SAVI catheters. There is a very limited guidance available in current literature [5] on the acceptable maximum dose to the heart. After the posterior catheter was turned-off in the original plan, the dose to heart was reduced after two fractions. The composite dose to 0.3% of heart volume was limited to 981 cGy as shown in Table 2. This case also demonstrates excellent dosimetry options offered by SAVI® to customize patientspecific treatment plans.
Conclusion
We have demonstrated that kV based Cone Beam CT Imaging available on modern linear accelerators can be used for verifying 3D alignment of SAVI applicator before HDR treatments are delivered. The technique provides additional confidence that the prescribed radiation dose is being delivered with conformal integrity to the original treatment plan. In departments that do not have traditional simulators or CT scanners in their departments, this technique allows for rapid verification of SAVI applicator without moving patient for HDR therapy.
Acknowledgements
We would like to acknowledge excellent training and support provided by Cienna Medical Staff as we developed this program in our center.
References
- Susan Hoover, Elizabeth Bloom and Sunil Patel. Review of Breast Conservation Therapy: Then and Now. International Scholarly Research Network Oncology. 2011: 1-13.
- Polger C, Major T, Fodor J et al. Accelerated partial-breast irradiation using High-dose interstitial brachytherapy: 12 year update of a prospective study. Radother Oncol. 2010; 94: 274-279.
- Nelson JC, Beitsch PD, Vicini FA, et al. Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial. Am J Surg. 2009; 198: 83-91.
- Benitez PR, Streeter O, Vicini F, et al. Preliminary results and evaluation of Mammosite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ: a phase II clinical study. Am Surg. 2006; 192: 427-433.
- Salih Gurdalli, Robert R. Kuske, Coral A Quiet and Mustafa Ozer. Dosimetric performance of Strut-Adjusted Volume Implant: A new single-entry multi catheter breast brachytherapy applicator. Brachytherapy. 2011; 10:128-135.