
Research Article
Austin J Radiat Oncol & Cancer. 2016; 2(2): 1022.
3-D Image-Based Radiotherapy Planning for Syed Interstitial HDR Brachytherapy in Patients with Parametrial Spread of Cervical Carcinoma
Mobit PN1,2, Packianathan S1 and Yang CL1*
¹Department of Radiation Oncology, University of Mississippi Medical Center, USA
²Cameroon Oncology Center, P O Box 1870, Douala, Cameroon
*Corresponding author: Yang CL, Department of Radiation Oncology, University of Mississippi Medical Center, 350 West Woodrow Wilson Ave., Jackson, MS 39213, USA
Received: September 23, 2016; Accepted: November 02, 2016; Published: November 04, 2016
Abstract
Objective: To investigate some practical issues involved in multi-fraction Syed interstitial HDR brachytherapy for patients with cervical carcinoma.
Methods: Between 8/2009 and 10/2010, eight such patients were treated at our facility with this technique. The high risk CTV (HRCTV), intermediate risk CTV (IRCTV), and organs at risk (OAR: rectum, bladder, sigmoid, and small bowel) were contoured on the planning CT images. All patients received 7 fractions of 4.2Gy prescribed to HRCTV, administered over 4 days. Planning objectives included: doses to OARs were kept below 80% of prescription dose, D90 for HRCTV = 90% of prescription dose, and V150 and V200 = 50% and 20%, respectively. OAR doses were evaluated using the dose to 2mL (D2 mL).
Results: An average of 2.4 treatment plans per patient was required to treat all fractions. The average dose to 2 mL (D2 mL) of the bladder was 63% ± 5% (1SD) of the prescription dose (29.4Gy). This value for the rectum was 64% ± 3.4%. The D2 mL values for sigmoid and small bowel were less than 40% of 29.4Gy. The D90 for HRCTV was on average 31.7Gy, whereas the D90 for IRCTV was on average 20Gy. V150 and V200 were on average 51% and 28%, respectively.
Conclusion: The implanted needles tended to move during patient transfers to and from the inpatient room to the treatment suite so pre-treatment CT scans should be performed daily and a new treatment plan should be generated if needle movements are significant.
Keywords: Syed Interstitial Brachytherapy; HDR; Cervical Carcinoma
Introduction
Many studies have suggested that High Dose Rate (HDR) brachytherapy is of comparable efficacy to Low Dose Rate (LDR) brachytherapy for patients with early to advanced stage cervical cancer [1-4]. This is in addition to providing other benefits such as reduced radiation dose to clinical personnel and customized anatomicbased planning for each applicator insertion. Many cancer centers have thus adopted HDR brachytherapy using vaginal cylinders, tandem and ovoids (T&Os), or tandem and ring (T&R) for their gynecologic cancer treatment programs. However, these applicators may not always optimally cover the lateral spread of cancer, where an interstitial implant should be considered [5].
The Cervical Cancer Brachytherapy Task Group Report was published [6] in 2012 by the American Brachytherapy Society (ABS) for general recommendations. Even though our research was performed before the publication of the report, we essentially followed their guidelines that dosimetry should be performed every time the applicators are inserted to assess the doses to the targets as well as the normal tissues. While several individual experiences have been reported for HDR brachytherapy based on solid applicators mentioned above, few such extensive studies have been reported for HDR interstitial brachytherapy for cervical cancer [1,3,7]. Although a number of studies have described and evaluated interstitial brachytherapy utilizing the LDR technique, the treatment planning in these cases did not typically involve the use of 3-D imaging dataset such as CT or MRI to assess the doses to organs at risk [8,9]. Additionally, needle movements for interstitial prostate HDR brachytherapy has been reported by many investigators in the literature [10,11]. Thus, our objective is to report in detail some of the practical issues, for instance, the frequency of reimaging and replanning involved in 3-D image-based treatment planning, and to investigate the clinical planning parameters important in multifraction Syed interstitial HDR brachytherapy for patients with locally advanced cervical carcinoma. Another motivation of this work is to evaluate the adequacy of dose coverage for lateral disease in the patient group.
Methods and Materials
Patient population
Between 8/2009 and 10/2010 eight patients were treated at our facility with Syed interstitial HDR brachytherapy for advanced cervical carcinoma. The average age of the patients was 51 years (age range 36 to 68 years). The distribution of their FIGO disease stages was as follows: IIB: 12.5%, IIIB: 50%; IVA: 25%; cuff recurrence: 12.5%. All patients already received 25 fractions of external beam radiation therapy (EBRT) at 1.8Gy per fraction to the whole pelvis followed by 3 fractions of 1.8Gy each delivered as a parametrial/ sidewall boost with a midline block. This study was approved by our institutional review board.
Operating room
Before the implant procedure, each patient underwent routine bowel preparation procedures in the morning of the procedure day. They were also kept on a clear liquid diet on the day prior to the procedure. Following induction of anesthesia, patients were positioned in the dorsal lithotomy position and a Foley catheter was asceptically placed with 7mL of contrast within the bulb and sterile draping of the operative field was completed. The cervix was visualized and 4 fiducial gold markers were inserted at the 3, 6, 9, and 12 o’clock positions on the cervix. We used the modified Syed- Neblett template for the brachytherapy needle placements. 18 to 21 flexible, plastic needles (Alpha-Omega Services Inc., Bellflower, California) were inserted into the cervix and parametria. The needles comprise a 17F flexi-guide with a female Luer adaptor and a stylet with a male Luer adaptor. A collar and O-ring were used with the template with the central core inserted into the vagina. Needles are implanted according to a tentative pre-plan that had been developed based upon the extent of disease identified at the time of diagnosis. This pre-plan is based on CT, MRI and clinical exams. The radiation oncologist would outline the volume that he would like to treat with the HDR procedures. Using this information, the physicist and the radiation oncologist would then mark the positions of the needles that would use. If more of the disease is on the left, then a few more needles would be implanted on the left side of the patient. After the needles had been implanted, the template holding the needles was then sutured flush to the perineum at 4 points. A rectal tube was also inserted into the rectum to aid in identifying the rectum on future CT scans. The patient was transferred to the post-anesthesia care unit and then transported by ambulance to the outpatient radiation oncology department, about a mile away from the main hospital.
CT simulation
At the outpatient radiation oncology department, the patients underwent a limited CT scan of the pelvis. Following the first evaluation CT scan, some needles were manually repositioned by the radiation oncologist if it was thought that they did not cover the extent of disease in the cranio-caudal direction. Once needle positions were deemed appropriate, the flexi-guide needles were glued in position at the external point where they passed through the template. Thereafter, a final CT scan was performed for brachytherapy treatment planning with slice thickness of 2mm. During any CT scanning, the plastic needles would have their individual stylets in place so as to help to be identified individually later on the CT scan.
Brachytherapy treatment planning and dose prescription
The CT data was then transferred to the Varian Brachyvision Brachytherapy Treatment Planning System for plan development. The high risk CTV (HRCTV), intermediate risk CTV (IRCTV), and organs at risk including rectum, bladder, sigmoid, and small bowel were all individually contoured. The Foley catheter balloon with the contrast media was also contoured. The contours were based on GEC-ESTRO contouring guidelines [12]. In accordance with GEC ESTRO guidelines [12], the HRCTV includes the gross tumor volume. HRCTV starts superiorly at the level of the uterine vessel where it abuts the cervical tissue. The volume includes the entirety of the cervix with coverage of the most inferior extent of vaginal disease as well as laterally to include the parametrial tissues should they be involved. Should uterine extension be visible, para-uterine tissue is also included. IRCTV is generated via 1 cm expansion beyond HRCTV with removal of volume that expanded into bladder, sigmoid colon, and the rectum. For stage IIB disease, coverage of the parametrial, uterosacral, and vaginal disease must also be included.
Following completion of the contouring, the needles were individually identified by the planning medical physicist. Because of their intrinsic flexibility, 2 or more of the flexi-guide plastic needles would sometimes overlap. Mis-identification of needles could have a significant dosimetric consequence and hence, each needle was always identified in the coronal, sagittal, and axial planes by two medical physicists prior to completion of the planning process. This constitutes part of the second check procedure implemented at our institution for this procedure. The prescription dose was 4.2Gy per fraction for 7 fractions for a total dose of 29.4Gy. Figure 1 shows the anterior-posterior and right lateral digitally reconstructed radiographs for an implanted Syed patient. It is apparent from the figure that these plastic needles would sometimes overlap and therefore increase the chances of mis-identification of needles.
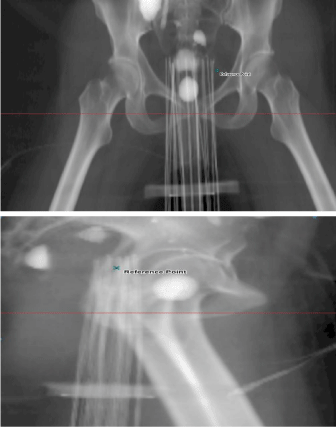
Figure 1: Anterior-Posterior and Right Lateral digitally reconstructed
radiographs of an implanted Syed patient generated with the Varian
Brachyvision treatment planning system.
Our planning objectives were to keep the D2 mL or the highest dose to 2mL of the organs at risk below 80% of the prescription dose, the D90 for the HRCTV = 90%, and to keep the V150 and V200 of the HRCTV to be less than 50% and 20%, respectively. We additionally required that the D90 for the IRCTV be greater than 65% of the prescription dose to ensure that the intermediate risk CTV gets adequate dose. These were institutional criteria that were developed based on our previous experience with high dose rate prostate implant brachytherapy. Another important requirement was that all source dwell positions should lie only within the HRCTV or IRCTV. We also restricted the dwell time for each dwell position to be at least 2 seconds. The Varian Brachyvision adaptive volumetric optimization program available within the standard Brachyvision Software Version 8.9 was used to generate the treatment plans. Optimizations involve manually adjusting the dwell times and positions of the radioactive source within HRCTV and IRCTV to meet our institution developed criteria. Initially each treatment plan was optimized with inverse planning objectives as described previously. The final plan was adjusted manually using isodose re-shaping and evaluation of the dose volume histograms. Once an acceptable plan was achieved and approved by the radiation oncologist, it was second-checked and verified by another medical physicist prior to the plan parameters being transferred to the after loader for treatment delivery. On the first day, the patient received a single treatment fraction before being returned via ambulance to the main hospital. She would return the following morning to receive two fractions, timed and delivered at least 6 hours apart. This process was repeated until all 7 fractions were administered.
CT simulations and treatment planning on 2nd, 3rd, and 4th days
Only one treatment was delivered on the first day. On days 2, 3, and 4, the patient was brought to the radiation oncology department by ambulance and underwent a CT scan. The patient was positioned as close to the first day set-up as possible so as to minimize fusion errors with the CT scan from the first day or the previous day CT scan whichever has the latest treatment plan. A quick review of the CT scan was performed in the CT console while the patient was still on the CT scan couch to determine if needles had migrated significantly in the cranio-caudal direction. If so, the needles were repositioned and the patient rescanned. The CT images were exported to Varian Brachyvision Treatment Planning System. Within Brachyvision, the CT data was co-registered with the previous day’s CT which has the latest treatment plan using the pixel-to-pixel image fusion algorithm. All the contours from this CT scan were copied into the new CT dataset and a detailed review of the contours was completed by the medical physicist and the radiation oncologist. The contours were modified by the radiation oncologist as needed. Then, the previous day’s brachytherapy plan (i.e. the plan was last used to treat the patient) was displayed on the new CT dataset for review. Evaluation of the treatment plan on the new CT scan to determine if a new treatment plan was needed was based on the target volume coverage and doses to organs at risk. One of the criteria was that D90 for the HRCTV should not be less than 90% and D2 mL or the highest dose to 2mL of the organs at risk should be below 80% of the prescription dose, especially for the rectum. A decision was then made as to whether a new treatment plan would be generated or to proceed using the previous day’s treatment plan as displayed on the new CT dataset. The patient then underwent appropriate treatment and rested in the department for the requisite 6 hours before her second treatment fraction for the day. Before the 2nd treatment fraction of the day, there was no CT scan and dosimetry evaluation performed with the assumption that there was not much needle movement involved during these six hours of waiting. However, before delivering the daily 2nd treatment fraction, the radiation oncologist will visually verify the integrity and consistency of the needles.
Results
In Table 1 we present the HRCTV and IRCTV measurements for each of the 8 patients. The HRCTV volumes varied from 10.6mL to 105.4mL, with an average of 69.4mL. The volumes of the IRCTV ranged from 64.0mL to 283.2mL. The mean volume of the IRCTV for all the patients with IRCTV was 173.6mL Only one patient out of eight did not have an IRCTV because the patient had a very large high risk CTV and a decision was made not to generate an IRCTV.
Patient number
High risk CTV volume (mL)
Intermediate risk CTV volume (mL)
1
45.5
79.8
2
59.5
117.2
3
64.8
205.4
4
105.4
n/a
5
86.6
231.4
6
10.6
64
7
93.5
234.5
8
93.5
283.2
Mean
69.4
173.6
Table 1: Volume of the high risk CTV and intermediate risk CTV for the 8 patients.
Table 2 shows the number of plans that were required to treat each patient. It is important to note that for all except one patient, at least 2 brachytherapy plans were necessary to completely treat the patient with the 7 HDR fractions on 4 separate days. On average though, 2.4 plans were generated per patient because the needle positions were sufficiently altered to invalidate the previous plan. For patient no. 2, however, a new plan had to be generated everyday due to significant needle movements. Table 2 also shows the number of implanted needles necessary to achieve our planning objectives. On average, 14 needles were used per treatment plan although 18-21 needles were actually implanted per patient.
Patient number
no. of needles used
no. of plans
1
13
2
2
14
4
3
14
2
4
18
2
5
13
3
6
12
3
7
15
1
8
16
2
Average
14
2.4
Table 2: Number of plans and needles needed to treat per patient.
Table 3 shows the dosimetric information for the HRCTV and the IRCTV. The D90 for the HRCTV ranged from the minimum prescription dose of 29.4Gy to a maximum of 33.9Gy (115%). The average value of D90 for the HRCTV was 31.7Gy or 108% of the prescription dose. In all the cases the radiation oncologist had specified that the HRCTV D90 must be at least 100% of the prescription dose.
Patient number
D90 (Gy) for HRCTV
D90 (Gy) for IRCTV
V150
V200
1
33.8
23.7
57.00%
28.00%
2
29.4
14.8
48.70%
24.60%
3
29.4
16.8
58.00%
36.00%
4
31.3
18.6
52.60%
23.80%
5
33.9
21.2
51.00%
23.00%
6
32.8
22.4
58.00%
27.00%
7
33
19.3
51.50%
22.50%
8
30
24.7
38.40%
16.50%
Average
31.7
20.2
51.90%
25.20%
Table 3: Average treatment parameters for the 8 Syed interstitial implant patients.
Patient number
Bladder (Gy)
Rectum (Gy)
Sigmoid (Gy)
Small Bowel (Gy)
1
20.3
19.5
6.5
6
2
17.4
23.4
5.3
8.3
3
7
16.8
7.7
7.3
4
22.2
13
8.3
6.7
5
22.1
21.4
21
22.6
6
23.4
22.1
21.6
21.8
7
22.5
15.4
8.4
5.6
8
14.6
19.7
8.4
8
Average
18.7
18.9
10.9
10.7
Table 4: Dose to 2 mL (D2 mL) for organs at risk for all patients in this study.
The V150 and V200 for the HRCTV was on average 52% (range 38.4 to 58%) and 25.2% (range 16.5 to 36%), respectively, which were somewhat higher than the desired planning objectives (50 and 20%, respectively).
For the IRCTV, the mean D90 was 20.2Gy or 68% (range 50 to 84%) of the prescription dose. Since the IRCTV encompasses a much larger volume than the central disease, the dose coverage was, to an extent, restricted by the number of needles we were able to position laterally. Also, as the needles are plastic, they tend to flex medially towards the central core whenever any lateral resistance is encountered.
The average dose to 2mL (D2 mL) of bladder was 63 ± 5% (1SD) of the prescription dose (29.4Gy). This value for the rectum was 64 ± 3.4% (1SD). The sigmoid and the small bowel D2 mL dose values were generally all less than 40% of the prescription dose. However for 2 patients (patients no. 5 and 6) the doses to 2mL of the sigmoid and small bowel were above 21 Gy despite aggressive attempts at dose reduction without compromising coverage of the HRCTV.
Discussion
Kirisits et al. [13] showed that by using the University of Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer, they could deliver adequate dose to a point 1.5cm lateral to the traditional point A. This meant that they are able to cover more lateralized disease than that can be treated effectively with the traditional T&Os or T&R applicators. The mean HRCTV in their patients was 44mL. The mean HRCTV of the patients in our study, however, was close to 70mL which is about 60% greater than the mean disease volume treated in their study. The largest volume we encountered was 105.4mL and we were able to treat disease extending up to 4 cm lateral to point A. So, the modified Syed-Neblett template allowed us to treat a much ‘wider’ tumor volume than that would have been possible with either T&R or T&Os applicator.
Given that majority of our patients exhibited locally advanced disease at presentation (IIB: 12.5%; IIIB: 50%; IVA: 25%), there were considerable residual visible or detectable disease after EBRT. As such, our HRCTV appears to be bigger than those reported in other studies. Despite this, we were able to obtain a D90 for HRCTV equal to at least the prescription dose. In order to minimize patient morbidity it is also necessary to minimize the volume of the high risk CTV receiving 150% or higher of the prescription dose. The mean V150 and V200 for the HRCTV in our patients was 52% and 25.2%. These parameters were clinically selected by the radiation oncologist but the actual upper and lower limits have not been established. A more achievable objective for the V150 and V200 may be 55% and 30%, respectively, but this will have to be established through clinical trials, expert committee review, or extension of data from other brachytherapy applications. We also note that for T&R or T&Os applications, the clinically defined HRCTV receiving 150% and 200% of the prescription dose would likely be much greater than with Syed interstitial plan as shown by Hsu et al. [5].
The traditional organs at risk for gynecologic brachytherapy using T&Os or T&R, or vaginal cylinder, are the rectum and bladder. The average dose to 2mL (D2 mL) for bladder and rectum was 63 ± 5% (1SD) and 64 ± 3.4% (1SD) of the prescription dose respectively. The sigmoid and the small bowel values were generally all less than 40% of the prescription dose. However, not monitoring the dose to these additional organs may lead to higher morbidity with HDR and in particular with HDR interstitial brachytherapy. For instance, in 2 of our patients (patients no. 5 and 6) the doses to 2mL of the sigmoid and small bowel totaled above 21Gy (71.4% of the prescription dose) despite aggressive attempts at dose reduction without compromising coverage of the HRCTV, which was due to the two patients’ unique anatomies. Furthermore, while all treatment doses represent the clinical weighing of benefits against risks, it should be noted that on occasion, higher doses to sigmoid colon and small bowel may have to be tolerated to reduce higher doses to the rectum and bladder. Such balancing acts benefit from multi-fraction HDR brachytherapy as higher doses to one specific area in one fraction may be balanced with lower doses to the same area in another fraction.
Our experience suggests that it is important in multi-fraction HDR interstitial brachytherapy treatments utilizing 3-D treatment planning a new treatment plan to be generated for each new day of treatment. An alternative, as we have demonstrated in this study, is to co-register a new CT scan with the CT scan that was used to generate the prior treatment plan for the patient. Individual structures could then be copied from the previous CT scan into this new CT scan and the old plan overlaid on the new CT images. If clinically appropriate, the old plan can then be used to treat the patient. Otherwise a new plan may be quickly generated. Superimposing each plan generated in this manner on the latest CT scan permits the clearest delineation of the doses to the HRCTV, IRCTV, and OARs.
Needle movement during multi-fraction prostate or cervical HDR brachytherapy is a serious issue and must be addressed for each patient. The effect varies from patient to patient and evaluating needle movement is not a worthwhile endeavor if the plan cannot be modified to accommodate the effect of the needle movement. In terms of similarity, both prostate and cervical cancer HDR interstitial brachytherapy patients will have the needles implanted in the operating room under general anesthesia while the treatment is then delivered within the shielded brachytherapy suite typically some distance from the surgical suite. Between 2 and 7 fractions are typically required to deliver the full prescription dose. Several studies evaluating interstitial HDR for prostate cancer have reported needle migration between fractions [10,11]. Studies of interstitial HDR brachytherapy for cervical cancer have also reported needle or applicator movement between treatment fractions [14-17]. In all these studies, the needle movement apparently affected the quality of the dose delivered between fractions. We assessed the needle displacement in eight patients, not in terms of each individual needle movement, but in their effect on the original plan objective; i.e., coverage of the target volumes (HRCTV and IRCTV) and all organs at risk (rectum, bladder, sigmoid and small bowel), and generated new treatment plans when necessary.
As interstitial HDR cervical brachytherapy is, like prostate HDR interstitial brachytherapy, delivered in fractions over a number of days, one would expect a somewhat similar range of movement of the needles. However, such movements will likely be larger if the patient had to be transferred a significant distance from her hospital room to the brachytherapy suite. The implanted needles are highly likely to move during patient transfers to and from the HDR suite; thus, we recommend that CT scans be repeated daily. Co-registering the treatment-day-CT scan with the last CT scan which was used to generate the last treatment used to treat the patient permitted us to evaluate needle movements and to determine if a new treatment plan was needed. Use of modern automatic fusion software intrinsic to the treatment planning software or an extrinsic scan merging software is a quick and easy way to determine the effect of any needle movement on the original plan objectives for not only the targets, but also the OARs. We can use these co-registered images from each day of treatment to display the composite dose distribution on the initial or any selected image set as shown in Figure 2.
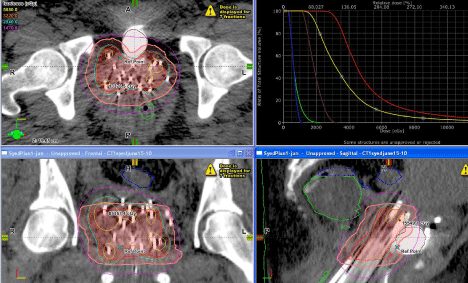
Figure 2: Composite dose distribution and dose volume histogram for a patient treated with Syed Interstitial technique.
Figure 3 shows the dose volume histograms for patient number 2 between the initial plan and the initial plan without any modification on the second day image set with modified structures. It can be seen that if the first day plan was used to treat the patient on the second day, the D90 for the high risk CTV would drop from 4.4Gy to 3.1Gy, which would not satisfy our planning goals specified above. This is the reason for re-planning for this case to improve the planning outcome.
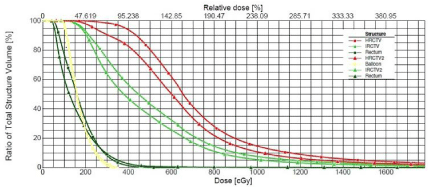
Figure 3: Comparison of the Dose Volume Histogram for patient number 2 between plan number 1 and the same plan on the 2nd day image set (without replanning).
HRCTV and IRCTV is the high risk CTV for the first day treatment while HRCTV2 and IRCTV2 is the high risk CTV for the second day CTV, respectively.
We have demonstrated from our clinical experience with treating the patients included in this study that it took an average 2.4 plans to treat an HDR Syed implant patient consisting of 7 fractions delivered over 4 days with patients being transported by ambulance between the hospital and the outpatient radiation oncology center (about two miles apart). It is important to note that after the needles had been implanted, the template holding the needles was then sutured flush to the perineum at 4 points. We also glued the flexi-guide needles at the external point where they passed through the template in order to hold them in position. These two procedures reduced individual needle movement and movement of the template as whole. This leads to fewer full re-planning for the majority of the patients. But despite this measure, we had to re-plan one of the patients in this study (patient number 2) daily before treatment. With a different patient population, we may expect some differences in the number of re-plans.
Conclusions
For our patients, between 18 and 21 needles were typically implanted but on average only 14 needles were used for treatment delivery. Because of needle movement, an average of 2.4 treatment plans per patient was generated to deliver all 7 fractions of the multifraction interstitial cervical HDR brachytherapy. The average dose to 2mL (D2 mL) of bladder was 63 ± 5% of the prescription dose (29.4Gy). This value for the rectum was 64% ± 3.4%. The dose to 2mL of the sigmoid and the small bowel were generally all less than 40% of the prescription dose. It is recommended CT/MRI images to be used for Syed interstitial HDR brachytherapy planning to adequately define the HRCTV, IRCTV, and OARs. The implanted needles tend to move during patient transfers to and from the HDR suite; thus, CT scans should be repeated at least once daily to determine if a new treatment plan needs to be generated. Use of automatic fusion software can simplify the daily evaluation of the new scan in comparison to the prior scans.
References
- Stewart AJ, Viswanathan AN. Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer. 2006; 107: 908- 915.
- Beriwal S, Bhatnagar A, Heron DE, Selvaraj R, Mogus R, Kim H, et al. High-dose-rate interstitial brachytherapy for gynecologic malignancies. Brachytherapy. 2006; 5: 218-222.
- Falkenberg E, Kim RY, Meleth S, De Los Santos J, Spencer S. Low-doseratevs high-dose-rateintracavitary brachytherapy for carcinoma of the cervix: The University of Alabama at Birmingham (UAB) experience. Brachytherapy. 2006; 5: 49-55.
- Saitoh J, Ohno T, Sakurai H, Katoh H, Wakatsuki M, Noda SE, et al. Highdose- rate Interstitial Brachytherapy with Computed Tomography-based Treatment Planning for Patients with Locally Advanced Uterine Cervical Carcinoma. J Radiat Res. 2011; 52: 490-495.
- Hsu IC, Speight J, Hai J, Vigneault E, Phillips T, Pouliot J. A comparison between tandem and ovoids and interstitial gynecologic template Brachytherapy dosimetry using a hypothetical computer model. Int J Radiat Oncol Biol Phys. 2002; 52: 538-543.
- American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix, Part II: high-dose rate brachytherapy.
- Isohashi F, Yoshioka Y, Koizumi M, Konishi K, Sumida I, Takahashi Y, et al. High-dose-rate interstitial brachytherapy for previously untreated cervical carcinoma. Brachytherapy. 2009; 8: 234-239.
- Syed AM, Puthawala AA, Abdelaziz NN, el-Naggar M, Disaia P, Berman M, et al. Long-term results of low-dose-rate interstitial-intracavitary brachytherapy in the treatment of carcinoma of the cervix. Int. J RadiatOncolBiol Phys. 2002; 54: 67–78.
- Kumar PP, Good RR,Jones EO. Dosimetry comparison between interstitial and intracavitary irradiation in the treatment of uterine cervix cancer. Radiat. Med. 1986; 4: 89-96.
- Damore SJ, Syed N, Puthawala AA, Sharma A. Needle displacement during HDR brachytherapy in the treatment of prostate cancer. Int J RadiatOncolBiol Phys. 2000; 46: 1205–1211.
- Kim Y, Hsu IC, Pouliot J. Measurement of craniocaudal catheter displacement between fractions in computed tomography-based high dose rate brachytherapy of prostate cancer. J. Appl Clin Med Phys. 2007; 8: 2415- 2427.
- Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006; 78: 67-77.
- Kirisits C, Pötter R, Lang S, Dimopoulos J, Wachter-Gerstner N, Georg D. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J RadiatOncolBiol Phys. 2005; 62: 901-911.
- Mullokandov E, Gejerman G. Analysis of serial CT scans to assess template and catheter movement in prostate HDR brachytherapy. Int JRadiatOncolBiolPhys. 2004; 58: 1063-1071.
- Jones N, Rankin J, Gaffney D. Is simulation necessary for each high-doserate tandem and ovoid insertion in carcinoma of the cervix?. Brachytherapy. 2004; 3: 120-124.
- Corso CD, Jarrio C, Nunnery EW, Ali AN, Ghavidel S, Rossi PJ, et al. Dosimetric and cost comparison of first fraction imaging versus fractional reimaging on critical organ dose in vaginal cuff brachytherapy. Pract Radiat Oncol. 2013; 3: 256-262.
- Damato AL, Cormack RA, Viswanathan AN. Characterization of implant displacement and deformation in gynecologic interstitial brachytherapy. Brachytherapy. 2014; 13: 100-109.