
Research Article
Austin J Radiol. 2023; 10(1): 1211.
Radiological Evolution of Patients with COVID-19 Pneumonia Treated with Low-Dose Radiotherapy
Gomez-Pena S1*, Martin Lores I1, Sanmamed N2, Fuentes M3, Vazquez M2, Bustos A1,4
1Diagnostic Imaging Department, Hospital Clínico San Carlos de Madrid, Spain
2Radiation Oncology Department, Hospital Clínico San Carlos de Madrid, Spain
3Preventive Department, Hospital Clínico San Carlos de Madrid, Spain
4Instituto de Investigación Clínico San Carlos (IdISSC). Fundación para la Investigación Biomédica Hospital Clínico San Carlos, Spain
*Corresponding author: Gomez-Pena SDiagnostic Imaging Department, Hospital Clínico San Carlos de Madrid, Calle del Prof Martín Lagos, S/N, 28040, Madrid, Spain
Received: December 22, 2022; Accepted: February 02, 2023; Published: February 09, 2023
Abstract
Background: The main objective of this work is to describe the findings and radiological evolution on chest CT of 27 patients with COVID-19 pneumonia after being treated with LD-RT in a prospective study. We also evaluated the interobserver agreement in assessing the extent of lung involvement.
Patients and Methods: 3 CTs were compared: day of treatment, one week later, and at 4-7 months. In each CT the following radiological findings were evaluated in each lobe: extension score (0: none, 0%; 1: minimal, 1-5%; 2: mild, 5-25%, 3: mild-moderate, 26-50%; 4: moderate, 51-75%; 5: severe, 76-100%), GGO, consolidation, crazy-paving pat-tern, subpleural lines, parenchymal bands, and pulmonary fibrosis.
Results: A statistically significant decrease in the number of affected lobes with consolidations and GGO was found between the second and third CT (p=0.023 and p=0.003, respectively) and between the first and third CT (p=0.012 and p=0.006). No significant changes were identified regarding the presence of fibrosis. There was a significant decrease of the extension score when comparing the three studies (1st vs 2nd p=0.029, 2nd vs 3rd p<0.001, and 1st vs 3rd p<0.001). A very good concordance was found in the evaluation of the extension score and the presence of consolidations, there was a moderate agreement when assessing the fibrosis and only a mild agreement on the GGO.
Conclusions: Our study suggests that patients with COVID-19 pneumonia treated with LD-RT show radiological improvement of consolidations and GGO on long-term follow-up chest CT, with no significant increase in pulmonary fibrosis identified.
Keywords: COVID-19; Radiotherapy; Pulmonary fibrosis; CT; Long-term follow-up
Abbreviations: LD-RT: Low-Dose Radiotherapy; COVID-19 Disease: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; CT: Computed Tomography; PTV: Planning Target Volume; Dmax: Maximal Dose; GGO: Ground Glass Opacities; IQR: Interquartile Ranges; ICC: Intraclass Correlation Coefficient
Introduction
Low-Dose Radiotherapy (LD-RT) has been shown to have an anti-inflammatory effect, modulating the inflammatory cascade,and reducing proinflammatory cytokines and acute-phase reactants (C-reactive protein, ferritin, lactate dehydrogenase or D-dimer). The LD-RT was used at the beginning of the 20th century as a treatment for pneumonia with several studies that suggested its potential efficacy, although its use was progressively abandoned after the introduction of antibiotics [1-3].
The COVID-19 disease (Coronavirus disease 2019) caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) emerged in Wuhan (China) in December 2019 and subsequently spread throughout the world [4]. In those first moments of the pandemic we found health systems unprepared for this health emergency and with the absence of effective treatments, which is why several researchers used LD-RT to treat patients with severe COVID-19 pneumonia, which presents a hyperimmune response that triggers a storm of cytokines [5-7].
The recommended dose in the initial phase of viral infection is 0.1-0.5 Gy, while that to eliminate cytokine-generating cells is 3-8 Gy, both doses well below the clinical dose used in the treatment of thoracic tumours [8]. Some of the described risks associated with LD-RT are induced heart disease or the development of radioinduced tumours, although with higher doses than those previously mentioned [9,10]. Another known risk is pulmonary fibrosis; for Rosen et al. the probability of producing pulmonary fibrosis with doses less than 30 Gy is less than 2% with conventional fractionation and less than 4% with accelerated fractionation, while for Tsujino et al. a pulmonary dose of 5 Gy is an independent and significant risk factor for the appearance of radiation pneumonitis [11,12]. Del Castillo et al. affirm that the treatment of COVID-19 pneumonia with a dose <1 Gy should not be a concern in the short or long term, since the dose in the LD-RT is far from the toxicity ranges [13]. To date, no studies have been published on possible pulmonary fibrosis caused by LD-RT treatment in patients with COVID-19 pneumonia.
The vast majority of published studies have focused on the radiological evolution of pulmonary involvement in the acute phase or during the first months of COVID-19 pneumonia [14-17]. Some authors have described the appearance of fibrotic pulmonary changes in Computed Tomography (CT) secondary to COVID-19 pneumonia after the resolution of the acute process, affecting approximately 30-40% of hospitalized patients [13-21].
The objective of this work is to describe the findings and radiological evolution on chest CT of patients with COVID-19 pneumonia after being treated with LD-RT and to evaluate the interobserver agreement in assessing the extent of lung affectation.
Patients and Methods
Study Design and Population
This study was approved by the Ethics Committee of our centre. It is a prospective and analytical study in which all surviving patients with COVID-19 pneumonia treated with LD-RT have been included from the total that had participated in phase 1-2 clinical trial of a single group (NCT-04420390) previously performed in our centre, and in whom a control chest CT was performed at 4-7 months due to persistent respiratory symptoms. The final results of the aforementioned clinical trial have already been published [5].
The inclusion criteria were: patients ≥50 years with a positive RT-PCR test for SARS-CoV-2 and with a chest imaging study compatible with bilateral pulmonary involvement and with a requirement for oxygen therapy.
Radiation Treatment
CT simulation was performed for treatment planning purposes. Patients were immobilized in supine position with a wedge-shaped mattress to perform a CT scan (Toshiba Aquilion LB 1800mm Computerized Tomography device, Toshiba corp., Tokyo, Japan. Clinical Target Volume (CTV) included both lungs. Planning Target Volume (PTV) was generated by adding 1 cm cranial, anteroposterior and lateral, and 2 cm caudal. Participants received 100cGy in a single fraction prescribed to the PTV. Dose planning goals were 80% of the dose received by >95% of the PTV volume and maximal dose (Dmax) <115%.
Treatment planning was carried out using a Three-Dimensional Conformed Radiotherapy technique (Eclipse v.15.6 Varian Medical Systems, Palo Alto, CA - USA), with two opposite anteroposterior beams and 6MV photons.
Our current work, therefore, includes all those patients who survived the aforementioned clinical trial, so the only exclusion criterion was death in the period between the performance of the chest CT 7 days post-radiotherapy and the CT control at 157 days on average.
Study Variables
All the data collected were obtained from the clinical records of the patients
Outcome Variables
• Primary outcome variables: extent of pulmonary involvement (extension score) and presence or absence of pulmonary fibrosis in chest CT studies (distortion of the lung architecture, reticulation, bronchial dilatations, and honeycombing).
Three chest CT scans of each patient were retrospectively analysed: pre-LD-RT (day 0) and post-LD-RT (mean day 7 and 157 days). In each of the CT studies, the following radiological findings were evaluated: presence of Ground Glass Opacities (GGO), consolidation, crazy-paving pattern, subpleural lines, parenchymal bands, and pulmonary fibrosis (distortion of pulmonary architecture, reticulation, bronchial dilations, and honeycombing). The distribution and predominance of the findings were also evaluated, as well as the predominant pattern in each lobe (A: normal; B: GGO; C: consolidation; D: bronchial dilations; E: fibrosis; F: reticulation; G: crazy-paving pattern) and the presence of fibrosis (yes/no). Radiological findings were defined according to the Fleischner Society guidelines (27). In addition, the distribution of the lesions was determined as central (internal 2/3 of the lung), peripheral (external 1/3), or diffuse. The presence of pleural effusion and its distribution (right, left, or bilateral), pneumothorax, pneumomediastinum, and air cysts (>2 cm) were also evaluated. Radiological findings were defined according to the Fleischner Society guidelines (27).
To assess the pulmonary extension of the disease, a semiquantitative score was calculated in each of the 5 lung lobes (0: none, 0%; 1: minimal, 1-5%; 2: mild, 5-25%, 3: mild-moderate, 26-50%; 4: moderate, 51-75%; 5: severe, 76-100%). The scores of the five lobes were added to obtain an overall score for each CT, between 0 to 25 (28).
Image Acquisition and Analysis
The LD-RT planning chest CT scans were performed on a 16-row scanner (Toshiba Aquilion LB 1800mm, Toshiba Corp., Tokyo, Japan) at the Radiation Oncology service. Successive chest CT studies at 7 and 157 days on average after LD-RT treatment were performed in the Radiodiagnostic service, with two sets of 64 rows of detectors depending on availability (Optima CT660, General Electric. New York, USA and Brilliance 64, Philips, Eindhoven, The Netherlands). The studies were done with the patient in the supine position, acquiring the entire lung parenchyma, from the vertices to the bases.
The analysis of the images was carried out on the workstations using the IMPAX 6.5.33 program (Agfa-Gevaert NV, Madrid, Spain). Each of the studies was reviewed both in the mediastinum window (350, 50 HU) and in the lung window (1500, -600 HU). The chest CT images were analysed independently by two thoracic radiologists with 27 and 5 years of experience (ABGC and IML, respectively), and discrepancies were jointly decided by consensus.
Statistical Analysis
Descriptive analyses are summarized as means with Standard Deviations (SD) and medians with interquartile ranges (IQR) for continuous characteristics with normal and non-normal distribution, respectively, and with frequencies and proportions for categorical characteristics.
Analysis of the Variables
The extension score variable was analysed as a quantitative variable. Due to the small sample size, a non-parametric test (Wilcoxon signed rank test) was used to evaluate the differences between the scores obtained in the three CTs. The McNemar test was used for qualitative variables.
To study the correlation between the two thoracic radiologists when determining the extension score, the Intraclass Correlation Coefficient (ICC) was used, while for the rest of the non-continuous variables the test used was the kappa coefficient.
For the sex- and age-stratified analysis of the extension score, we first calculated the absolute difference in each patient’s score when comparing the three CT studies (1st vs 2nd CT, 1st vs 3rd and 2nd vs 3rd). With this new non-normal distribution variable, we compared those obtained for each of the sexes (men vs. women) and each of the age ranges (≤65 vs >65) using the nonparametric Mann-Whitney U test. The choice of 65 years as the cut-off point for age was an arbitrary choice as it seemed more demographic and epidemiological.
For all tests, a significance value of 5% was accepted. Data processing and analysis were performed using the statistical package SPSS 15.0.
Results
The total sample of this study was 27 patients of the initial 41 who had been included in the clinical trial, which represents a mortality of 34.1%. The 27 patients had a median age of 67.1±12.9 years (IQR 50-90), 18 males (66.7%). Two of the 27 patients did not have CT on the seventh day post-LD-RT due to hemodynamic instability. The median time between LD-RT and the third CT scan was 157 days (range 72-236).
Assessment of Radiological Findings
A statistically significant decrease in the number of affected lobes with consolidations was found between the second and third CT (p=0.023) and between the first and third CT (p=0.012), although this significance was not observed between the first and second study (p=0.57). Thus, in the first CT study we observed 35/135 affected lobes with consolidation (26%), while in the last CT we only observed 5/135 (3.7%). Regarding the number of lobes affected by GGO, a statistically significant decrease was also seen when comparing the second study with the third (p=0.003) and the first with the third (p=0.006), but not when comparing the first. Study with the second (p=0.581). Thus, in the first study 122/135 affected lobes (90.4%) are observed, and in the third CT 74/135 (54.8%) (Table 1. Figures 1 y 2).
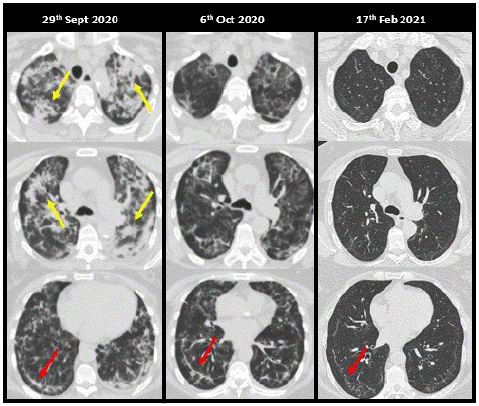
Figure 1: A 68-year-old woman, a kidney transplant recipient, with clinical symptoms of COVID-19 of one week’s evolution, was admitted for bilateral pneumonia requiring oxygen therapy on 09/18/2020. In the first days of admission, she presented clinical worsening, with radiological and high-concentration oxygen requirements, despite a dose of 400 mg of tocilizumab and high-dose steroids for 18 days. For this reason, it was decided to administer LD-RT (1 Gy) on 09/29/2020. Clinical improvement with a progressive decrease in oxygen requirements on successive days. Extensive bilateral patchy consolidations (yellow arrows), most evident in the upper lobes and upper segments of the lower lobes, are identified on the LD-RT planning CT, alternating with other GGO. On the second CT, a clear radiological improvement is identified with replacement of most of the consolidations by GGO. In the control CT 4.5 months after the LD-RT, a practical resolution of the pulmonary involvement is identified with the persistence of minimal reticulation and subpleural lines (red arrows) in the lower lobes.
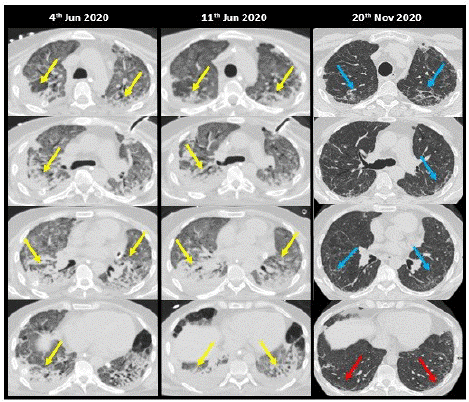
Figure 2: A 56-year-old male, with no relevant medical history, was admitted for bilateral SARS-CoV-2 pneumonia, treated with hydroxychloroquine and lopinavir/ritonavir. Poor clinical evolution with an increasing need for oxygen supply with mechanical ventilation and prone position. Boluses of methylprednisolone were administered without response and he was transferred to the ICU where orotracheal intubation was performed for invasive mechanical ventilation. The patient developed cor pulmonale, multiple respiratory complications, bacteremia, and pneumothorax secondary to barotrauma. It is decided to treat the patient with LD-RT (1 Gy). Progressively the patient improves clinically. LD-RT planning CT of the lung shows extensive areas of GGO attenuation in the upper lobes and consolidations in both lower lobes (yellow arrows) and mild pleural effusion. No significant changes were identified on CT at 7 days, except for a slight increase in bilateral pleural effusion. In the control CT at 5.5 months, a clear radiological improvement of the pulmonary consolidations and a bilateral interstitial pattern (blue arrows) predominantly in the upper lobes with reticulation, subpleural lines (red arrows), and bronchial dilatations are observed.
Age
Sex
ICU
Days until LD-RT
Total days of admission to hospital
CTs
Lobes affected by consolidation 1st CT
Lobes affected by consolidation 3rd CT
Lobes affected by GGO 1st CT
Lobes affected by GGO 3rd CT
Fibrosis 1st CT
Fibrosis 3rd CT
Age
1
86
Male
No
27
38
3
1
1
5
2
1
1
2
90
Male
No
22
31
3
2
0
5
3
0
0
3
53
Male
No
52
60
3
0
0
5
8
1
1
4
58
Female
No
64
80
3
1
0
5
2
1
1
5
55
Male
No
85
106
3
0
0
5
0
1
0
6
64
Male
Yes
79
106
3
0
0
5
2
0
0
7
56
Male
Yes
74
154
3
0
0
5
0
1
1
8
75
Male
No
93
125
3
0
0
4
0
1
1
9
63
Male
Yes
118
155
3
0
0
5
5
1
1
10
82
Male
No
4
9
3
0
0
5
5
0
0
11
61
Female
Yes
19
48
3
3
0
5
0
0
0
12
67
Female
No
12
20
3
3
0
5
0
0
0
13
83
Male
No
15
23
2
1
0
0
0
0
0
14
83
Male
No
13
33
3
0
0
3
0
1
1
15
54
Male
Yes
25
103
2
2
3
5
0
0
0
16
67
Male
No
58
64
3
0
0
5
3
1
1
17
62
Male
No
31
39
3
0
0
5
5
0
0
18
85
Female
No
14
46
3
4
1
5
2
0
1
19
52
Male
No
46
54
3
0
0
5
5
0
0
20
59
Male
No
11
18
3
5
0
0
5
0
1
21
62
Female
No
27
31
3
3
0
5
5
1
1
22
83
Male
No
17
24
3
1
0
5
0
0
0
23
90
Female
No
22
32
3
3
0
5
5
0
0
24
50
Female
No
14
65
3
0
0
5
5
0
0
25
84
Male
No
17
25
3
4
0
5
5
0
0
26
97
Female
No
8
21
3
0
0
5
5
0
1
27
82
Female
No
12
20
3
2
0
5
5
0
0
Table 1: Demographic and radiological features.
Regarding the presence or absence of pulmonary fibrosis, no significant changes were identified between the studies, with 12 patients (44%) presenting fibrosis in the first CT and 14 (52%) in the last study. When analysing more specifically each one of the parameters that define pulmonary fibrosis, no significant changes were identified either, except in reticulation, in which a significant increase was observed when comparing the first CT with the third (p<0.001) and the second with the third (p<0.001).
Regarding the extent of lung involvement (extension score) we found a significant decrease when comparing the three studies: the first CT with the second (p=0.029), the second with the third (p<0.001), and the first with the third (p<0.001) (Table 2).
Patients
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
Mean ± Standard deviation (range)
1 CT
25
17
23
23.5
25
25
24.5
19
12.5
21.5
21.5
22.5
9
10
25
22.5
15.5
22.5
18
15
18
16
15.5
25
19.5
21
17
19.61 ± 4.69
2 CT
25
17
23
23
10
25
25
16.5
24.5
20
18.5
15.5
11
17
16.5
22.5
17.5
14
16
16
15.5
25
19
18.5
15.5
18.68 ± 4.40
3 CT
7
6.5
10
23
12.5
5
12.5
22.5
14
21
3.5
7
3
6
24.5
6
10
13.5
12.5
10
10
9.5
10
15
15
13
8.5
11.52 ± 5.82
Table 2: Extension score of pulmonary findings for each CT.
In addition, we analyzed whether the extension score behaved differently between men and women or according to age (Table 3). In both sexes a similar profile is observed in the change of the extension score. When comparing the three studies with each other according to sex, we observed that all the changes in the extension score are statistically significant, except in the male sex the scores between the first and second CT. When we analyzed the extension score according to age, we also observed that there were no statistically significant differences by age. However, when analyzing each age group separately, we did observe statistically significant differences in the scores, except between the first and second CT in the youngest group.
1st CT
2nd CT
1st vs 2nd CT
1stvs 3rd CT
2ndvs 3rd CT
Men
19.06 ± 5.28
18.56 ± 4.86
11.53 ± 6.1
p = 0.326
p = 0.001
p = 0.003
Women
20.72 ± 3.19
18.89 ± 3.71
11.5 ± 5.57
p = 0.028
p = 0.008
p = 0.012
Men vs Women
p = 0.142
p = 0.395
p = 0.91
=65
20.89 ± 4.5
19.83 ± 5.1
12.5 ± 5.1
p = 0.341
p = 0.003
p = 0.004
>65
18.43 ± 4.72
17.62 ± 3.52
10.61 ± 5.77
p = 0.025
p = 0.002
p = 0.007
=65 vs >65
p = 0.472
p = 0.846
p = 0.479
Table 3: Comparison of extension score by sex and age (mean ± standard deviation).
Two-thirds of the patients presented a diffuse distribution of lung involvement on the first CT (18/27, 66.7%), a percentage that decreased on the second CT (13/25, 52%) and was less than one-third of the patients. patients in the last CT (6/27, 22.2%). Exactly the opposite occurred with the peripheral distribution, which occurred in just one third of the patients in the first CT (9/21, 33.3%) and in the last one it represented more than two thirds (21/27, 77.8%). No patient had a central distribution. In addition, significant changes were identified concerning the distribution when comparing the second CT with the third (p<0.001) and the first with the third (p<0.001).
In the first CT, 12 patients presented pleural effusion while in the third only 4 patients presented effusion. In one patient a pneumothorax was observed on the first CT that resolved in the second study. On the other hand, three patients presented pneumomediastinum in the first CT, and in two of them it had been resolved in the second study. Three patients presented pulmonary cysts larger than 2 cm, one patient had to undergo surgery because they caused recurrent pneumothorax.
Evaluation of Interobserver Agreement in the Assessment of Pulmonary Involvement
A very good concordance was found between the two readers in the evaluation of the extension score of the pulmonary affectation, with an intraclass correlation coefficient of 0.86 for the first study, 0.81 for the second, and 0.9 for the third study. Very good agreement was also seen when assessing the presence of pulmonary consolidations (number of affected lobes) with a weighted κ of 0.96, 0.72, and 0.88 for the first, second, and third CT, respectively.
However, this agreement was not as good in assessing the number of lobes affected by the presence of GGO with a weighted κ of 0.28, 0.63, and 0.19 for the first, second, and third CT scans, respectively.
For the assessment of the presence of fibrosis (yes/no), there was a moderate-significant agreement: κ = 0.71 in the first CT, κ = 0.5 in the second, and κ = 0.47 in the last.
The rest of the parameters are represented in (Table 4).
ICC
Kappa
Agreement(%)
Weighted Kappa
Agreement (%)
Weighted Kappa
Agreement (%)
Extension score 1CT
0.9
Crazy-paving pattern 1CT
0.54
74.07
Extension score 2CT
0.8
Crazy-paving pattern 2CT
0.52
79.17
Extension score 3CT
0.9
Crazy-paving pattern 3CT
1
96.3
Distribution 1CT
0.62
81.45
Subpleural lines 1CT
0.5
59.26
Distribution 2CT
1
100
Subpleural lines 2CT
0.45
48
Distribution 3CT
0.55
77.78
Subpleural lines 3CT
0.6
51.85
Consolidation 1CT
0.96
74.07
Parenchymal bands 1CT
0.41
51.85
Consolidation 2CT
0.72
72
Parenchymal bands 2CT
0.32
56
Consolidation 3CT
0.88
92.59
Parenchymal bands 3CT
0.34
66.67
GGO 1CT
0.28
74.07
Reticulación 1CT
0.39
55.56
GGO 2CT
0.63
72
Reticulación 2CT
0.32
56
GGO 3CT
0.19
59.26
Reticulación 3CT
0.53
51.85
Fibrosis 1CT
0.71
85.19
Bronchiectasis 1CT
0.69
44.44
Fibrosis 2CT
0.49
76
Bronchiectasis 2CT
0.64
44
Fibrosis 3CT
0.47
77.78
Bronchiectasis 3CT
0.45
33.33
Table 4: Interobserver agreement of radiological findings on CT.
Discussion
In our study, a clear radiological improvement was found in the pulmonary consolidations and the GGO pattern, as well as the COVID-19 pneumonia extension score, between the first and the last chest CT after LD-RT. However, no significant changes were found in terms of the signs of pulmonary fibrosis between the different radiological studies.
The radiological findings that we have found in chest CT studies in patients with COVID-19 pneumonia treated with LD-RT are consistent with those described in the literature: GGO pattern isolated or associated with consolidations and interstitial involvement, predominantly located in the lower lobes [2,3,14,28]. However, in the first two CT studies (days 0 and 7 post-LD-RT), the predominant distribution was diffuse versus peripheral, which could be explained by the fact that all our patients had a severe disease that had progressed both clinically and radiologically. The distribution showed good inter-observer agreement [3,14,28].
There was a statistically significant decrease in pulmonary consolidations between the first CT and the last (26% of affected lobes in the first CT and 3.7% in the last) and not between the first two, which is also consistent with what has been described. previously reported in the literature: pulmonary consolidations have a peak incidence between days 9 and 13 from the onset of symptoms and subsequently resolve slowly and are more frequent and extensive in patients with severe disease [14,16,29].
In our study, we have also observed a progressive decrease in the extensive GGO areas compared to the acute phase (90.4% of the lobes affected in the first study and 54.8% in the last), which is consistent with the literature [14,16,29]. However, the age and prolonged stay in the ICU of some patients could be related to the persistence of GGO found in our study: in 66.7% of the patients (18/27) and 54.8% of the affected lobes (74/135) persist in the last CT, despite the fact that they had significantly decreased compared to the first CT. This persistence of GGO in discharged patients has already been mentioned in some studies, and could even appear in more than half of the patients, as in our study [22,29-31].
For the evaluation of the extension of pulmonary involvement by SARS-CoV-2, several different methods have been described in the literature, both for chest radiography and chest CT [22,29,30]. The score used by us was described by Pan et al. in February 2020 and later by Li et al., Francone et al., and Liu et al. [15,16,28,29]. In general, it is observed that there is a high concordance between the evaluators when using them, which is consistent with our study. Therefore, this score could be of interest for use in routine clinical practice for the assessment of the extent of other pulmonary pathologies. In addition, in our study, the vast majority of patients had a very high score in the first two studies, which decreased in the last CT. The extension score did not show statistically significant differences by age or sex, which may be due to the fact that the patients were very severe and generally older.
Low concordance among readers was observed for some radiological findings such as GGO, crazy-paving pattern, or reticulation. In our opinion, this was mainly due to the low quality of some studies for various reasons, which made it difficult to assess the subtler radiological findings such as GGO or reticulation. An important reason is that the first CT scan was a study performed on a 16-row scanner located in the Radiation Oncology department for treatment planning. In addition, the clinical situation of the patients was generally severe or had poor respiratory capacity, so the patient was not very cooperative in performing inspiration correctly or in remaining still. Finally, it is possible that in the case of GGO there was a discrepancy in classifying it as such or as consolidation. On the other hand, less subtle and easier to assess radiological findings such as consolidation did show good interobserver agreement.
During the last two years, there have been multiple descriptions in the literature of pulmonary involvement with CT in the acute phase of COVID-19 pneumonia or during the evolution of the disease [14-17]. The appearance of pulmonary fibrotic changes after the resolution of the acute process has been described in up to 30-40% of hospitalized patients [18-26]. This fibrosis is secondary to alveolar cell damage, fibroblast persistence, and excessive collagen deposition that is accompanied by destruction and alteration of lung architecture [23].
In our work, we have found a higher proportion of fibrosis in patients with COVID-19 pneumonia treated with LD-RT than that found in other studies, both in the first chest CT, 44% (compared to 33.9% described by Zhou et al. and 17.5% by Pan et al.), as in evolutionary CT, 52% (compared to 39% described by Wei et al., 30% by Vasarmidi et al. and 25% by Lerum et al.) [22,24,29,32,33]. These findings could be explained because in our case only moderate-severe patients were included, some of them with prolonged stays in the hospital or admitted to the ICU, while in the aforementioned studies the heterogeneity of the sample was greater.
No significant changes were found in the presence of fibrosis during follow-up. They do not seem to be resolved in the short-medium term, but neither do they seem to progress significantly. Zhu et al. and Pan et. to the. affirm that, although these findings may already be visible in the acute phase of the disease, they are more frequent in the advanced stages [29,32]. These studies are supported by autopsy reports [26,34]. In a review by Ojo et al. The different risk factors for the appearance of pulmonary fibrosis after SARS-CoV-2 infection are described: advanced age, severe illness, prolonged length of stay in the ICU and mechanical ventilation, smoking, and chronic alcoholism [35]. In our study, only patients over 50 years of age have been included, some of whom had a prolonged hospital stay in the ICU with mechanical ventilation. Wei et al. affirm also that those patients with advanced age, a higher score in the extension score of the radiological findings in CT, longer hospital stays, and admission to the ICU, are more prone to pulmonary fibrosis [24].
In terms of absolute agreement when assessing fibrosis as a whole, we observed good agreement between the two readers. However, when we analyse each of the radiological findings that form part of the fibrosis (bronchiectasis, reticulation, or parenchymal banding), the agreement is not as good as it was expected. Thus, we can state that fibrosis is best assessed as a set of radiological findings, rather than assessing each of these findings individually.
Based on the above, it could be inferred that LD-RT has not produced a progression of pulmonary fibrosis in our series, since it was already present in the first CT studies and has not progressed significantly. New studies with larger samples are needed to support these claims.
This study has limitations. First, the sample size (n=27) is relatively small and the patients included in the study had a mean age of 64 years and clinically treated COVID-19 pneumonia without improvement, which limits the generalizability of the results. An important limitation is the heterogeneity in the time range of the last CT, so the radiological changes, which are highly dependent on time, could be biased. Finally, new controls with CT would be necessary to assess the evolution of the long-term radiological findings in these patients, since our study presents a relatively short follow-up. However, this study has important advantages such as the fact that the patients were treated homogeneously within a prospective study and it is, to our knowledge, the first study that evaluates the CT of patients treated with LD-RT.
Conclusion
Our study suggests that patients with COVID-19 pneumonia treated with LD-RT show clear radiological improvement of consolidations and GGO on long-term follow-up chest CT. However, LD-RT does not appear to significantly increase pulmonary fibrosis. On the other hand, as in previous studies, interobserver agreement was very good in assessing the extension score of pulmonary involvement.
References
- Calabrese EJ, Dhawan G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med. 2013; 86: 555-70.
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020; 34: 101623.
- Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT Findings in Patients With Coronavirus Disease 2019 and Its Relationship With Clinical Features: Invest Radiol. 2020; 55: 257-61.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients in-fected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020; 395: 497-506.
- Sanmamed N, Alcantara P, Gómez S, Bustos A, Cerezo E, Gaztañaga M et al. Low-dose Radiation Therapy in the Management of COVID-19 Pneumonia (LOWRAD-Cov19). Final results of a prospective phase I–II trial. Radiotherapy and Oncology. 2022; 171: 25-29.
- Ameri A, Rahnama N, Bozorgmehr R, Mokhtari M, Farahbakhsh M, Nabavi M, et al. Low-Dose Whole-Lung Irradiation for COVID-19 Pneumonia: Short Course Results. Int J Radiat Oncol. 2020; 108: 1134-9.
- Hess CB, Buchwald ZS, Stokes W, Nasti TH, Switchenko JM, Weinberg BD, et al. Low-dose whole-lung radiation for COVID-19 pneumonia: Planned day 7 interim analysis of a registered clinical trial. Cancer. 2020; 126: 5109-13.
- Li JJ. Mitigating Coronavirus-Induced Acute Respiratory Distress Syndrome by Radiotherapy. iScience. 2020; 23: 101215.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med. 2013; 368: 987-98.
- McKeown SR, Hatfield P, Prestwich RJ, Shaffer RE, Taylor RE. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol. 2015; 88: 20150405.
- Rosen II, Fischer TA, Antolak JA, Starkschall G, Travis EL, Tucker SL, et al. Correlation between Lung Fibrosis and Radiation Therapy Dose after Concurrent Radiation Therapy and Chemotherapy for Limited Small Cell Lung Cancer. Radiology. 2001; 221: 614-22.
- Tsujino K, Hashimoto T, Shimada T, Yoden E, Fujii O, Ota Y, et al. Combined Analy-sis of V20, VS5, Pulmonary Fibrosis Score on Baseline Computed Tomography, and Patient Age Improves Prediction of Severe Radiation Pneumonitis After Concurrent Chemoradiotherapy for Locally Advanced Non–Small-Cell Lung Cancer. J Thorac Oncol. 2014; 9: 983-90.
- Castillo RD, Martinez D, Sarria GJ, Pinillos L, Garcia B, Castillo L, et al. Low-dose radiotherapy for COVID-19 pneumonia treatment: case report, procedure, and literature review. Strahlenther Onkol. 2020; 196: 1086-93.
- Kong M, Yang H, Li X, Shen J, Xu X, Lv D. Evolution of chest CT manifestations of COVID-19: a longitudinal study. J Thorac Dis. 2020; 12: 4892-4907.
- Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020; 30: 6808-17.
- Liu J. Clinical and radiological changes of hospitalised patients with COVID-19 pneumonia from disease onset to acute exacerbation: a multicentre paired cohort study. Eur Radiol. 2020; 30: 5702-8.
- Wang YC, Luo H, Liu S, Huang S, Zhou Z, Yu Q, et al. Dynamic evolution of COVID-19 on chest computed tomography: experience from Jiangsu Province of China. Eur Radiol. 2020; 30: 6194-203.
- Fang Y, Zhou J, Ding X, Ling G, Yu S. Pulmonary fibrosis in critical ill patients recovered from COVID-19 pneumonia: Preliminary experience. Am J Emerg Med. 2020; 38: 2134-8.
- Gentile F, Aimo A, Forfori F, Catapano G, Clemente A, Cademartiri F, et al. COVID-19 and risk of pulmonary fibrosis: the importance of planning ahead. Eur J Prev Cardiol. 2020; 27: 1442-6.
- Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis. 2021; 21: 72.
- Kayhan S, Kocakoç E. Pulmonary Fibrosis Due to COVID-19 Pneumonia. Korean J Radiol. 2020; 21: 1273-1275.
- Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021; 57: 2003448.
- Ahmad Alhiyari M, Ata F, Islam Alghizzawi M, Bint I Bilal A, Salih Abdulhadi A, Yousaf Z. Post COVID-19 fibrosis, an emerging complication of SARS-CoV-2 infection. IDCases. 2021; 23: e01041.
- Wei J, Yang H, Lei P, Fan B, Qiu Y, Zeng B, et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J X-Ray Sci Technol. 2020; 28: 383-9.
- Barisione E. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2020; 478: 471-85.
- Rogliani P, Calzetta L, Coppola A, Puxeddu E, Sergiacomi G, D’Amato D, et al. Are there pulmonary sequelae in patients recovering from COVID-19? Respir Res. 2020; 21: 286.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology. 2008; 246: 697-722.
- Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia: Invest Radiol. 2020; 55: 327-31.
- Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020; 295: 715-21.
- Shah AS, Wong AW, Hague CJ, Murphy DT, Johnston JC, Ryerson CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021; 76: 402-4.
- Liu D, Zhang W, Pan F, Li L, Yang L, Zheng D, et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020; 21: 125.
- Zhou S, Zhu T, Wang Y, Xia L. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol. 2020; 30: 5446-54.
- Vasarmidi E, Tsitoura E, Spandidos D, Tzanakis N, Antoniou K. Pulmonary fibrosis in the aftermath of the Covid-19 era (Review). Exp Ther Med. 2020; 20: 2557-2560.
- Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020; 33: 1007-14.
- Ojo AS, Balogun SA, Williams OT, Ojo OS. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm Med. 2020; 2020: 1-10.