
Case Report
Austin J Radiol. 2023; 10(2):1214.
A Case Report of Progressive Multifocal Leukoencephalopathy in Pediatrics: When and How to Think About it?
Yahya El Harras*; Kaouthar Sfar; Rachida Chehrastane; Nazik Allali; Latifa Chat; Siham El Haddad
Pediatric and Gynecology Radiology Department, Children’s Hospital, University MOHAMMED V, Rabat Morroco
*Corresponding author: Yahya El Harras Pediatric and Gynecology Radiology Department, Children’s Hospital, University Mohammed V, Rabat Morocco. Email: elharrasyahya@gmail.com
Received: April 25, 2023 Accepted: May 19, 2023 Published: May 26, 2023
Abstract
Progressive Multifocal Leukoencephalopathy (PML) is a life-threatening demyelinating brain disease, usually caused by reactivation of a rare opportunistic infection with JC virus. This pathology is strongly associated with immunosuppressed states, with primary PML developing in an immunocompetent patient is very rare. Imaging has an important role in orientating the diagnosis. We report the case of an 8 years old girl, who was on chemotherapy, and suffered neurologically. Her MRI showed PML lesions. Through this case, we provide a review of the literature about this rare pathology to help clinicians and radiologists evoke it.
Keywords: Multifocal; Leukoencephalopathy; PML; MRI; Demyelinating
Introduction
Progressive multifocal leukoencephalopathy (PML) is a very rare demyelinating disease of the central nervous system due to reactivation of the JC virus (John Cunningham). It targets oligodendrocytes in the context of immunodepression, especially HIV-infected patients. Diagnosis combines a bundle of clinical, radiological and biological arguments (positive PCR in the CSF) or histopathological findings. Imaging shows the involvement of subcortical white matter in early stages with no enhancement after Gadolinium. We report the case of an 8-year old girl, who was diagnosed with T-lymphoblastic lymphoma who was on intensive chemotherapy. She presented to our department for an MRI due to neurological symptoms. PML diagnosis was then evoked.
Case Report
Our patient, an 8 year-old-girl, had a family history of a brother who died from leukemia associated to brain cancer. She was diagnosed two months ago with a T-lymphoblastic lymphoma, and was on intensive chemotherapy. Her family noticed some incoherent and nonsensical speech at times with agitation and irritability. Her oncologist referred her for altered mental status to our radiology department for brain MRI. The latter showed T2 and FLAIR bilateral non symmetrical hyperintensities affecting sub cortical white matter in both the supra and infra tentorial areas (Figure 1). The lesions didn’t show a restriction in diffusion weighted images and no enhancement after Gadolinium administration was noted (Figure 2). We evoked PML lesions and referred her to the pediatric oncology department for urgent medical care. She underwent a lumbar puncture and the qPCR for JCV returned positive, which further strengthened our diagnosis. The infant was started on antiretroviral therapy but unfortunately died 4 months later.
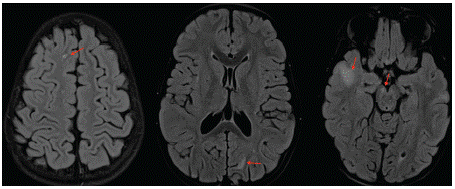
Figure 1: Axial FLAIR WI showing the bilateral non symmetrical hyperintensities of the sub cortical white matter (red arrows) noted in the supra tentorial area and in the right cerebral peduncle.
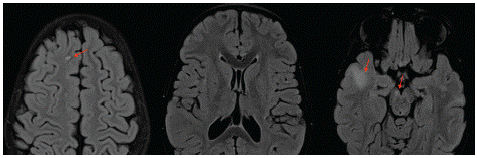
Figure 2: Axial T2 and DWI (A and B) showing the area of T2 hyperintensity subcortical white matter with no restriction in diffusion (blue arrows). Axial FLAIR (C) and T1-WI after Gadolinium administration (D) showing the absence of enhancement (red arrows).
Discussion
Progressive Multifocal Leukoencephalopathy (PML) is a demyelinating pathology, resulting from a subacute opportunistic infection caused by JC polyomavirus. This virus was first isolated from autopsied brain tissue from a patient named John Cunningham (JC), and it infects oligodendrocytes causing demyelination in immunocompromised patients (especially AIDS patients).
Being a ubiquitous virus, it circulates widely in the environment, primarily in sewage. More than 85% of the adult population worldwide has antibodies against JCV. We admit that probably asymptomatic infection is acquired in childhood or adolescence. It the remains latent until the virus is reactivated. In the case of immunodepression, the reactivated JCV can become neurotropic, infecting oligodendrocytes, and thus causing this progressive demyelinating encephalopathy [1]. Nevertheless, its incidence in the non-HIV setting is increasing, such as post-transplant population, in case of leukaemia, solid organ malignancies or when a chemotherapy is instaured [2].
Clinically, it manifests with various neurological symptoms, in particular an altered mental status, motor deficits or even limb and gait ataxia. It usually spares the optic nerve and the spinal cord. Seizures are also a possible manifestation as this disease can also involve the grey matter in later stages [3,4].
CT-SCAN may show asymmetric focal zones of hypodensities involving the subcortical and deep periventricular white matter, not enhancing after contrast administration [5].
The diagnosis key in MRI is multifocal demyelination subcortical white matter areas in hyperintense T2 and FLAIR, extending towards the deep white matter (peri ventricular). Usually, grey matter remains spared until advanced stages. Lesion are usually bilateral, but non symmetrical without any mass effect or enhancement after Gadolinium administration. We noted that the subcortical U-fibers are the most involved location with a predilection for the parieto-occipital regions and thalami [6]. In Diffusion Weighted Images (DWI), newer lesions have restricted diffusion to the periphery. However, there’s no restriction in the oldest ones. Recently, it has been reported if enhancement is present, it may be associated with improved survival and a better prognosis [5]. The second most common location is the posterior fossa white matter (in particular the middle cerebellar peduncles) [1].
Differential diagnosis includes other demyelinating diseases such as Multiple Sclerosis (MS), Acute Disseminated Encephalomyelitis (ADEM) and HIV-encephalitis [7]. The latter is also referred as HIV-Associated Dementia (HAD), and on imaging it is characterized by atrophic and symmetric abnormalities of the periventricular or diffuse white matter; usually sparing the subcortical U-fibres. Early MS disease can also be misdiagnosed as PML, but usually periventricular location or well-defined borders is in favor of new MS lesions (due to the fact that PML usually affects subcortical white matter and may evolve to periventricular location) [3]. ADEM is also a possible differential diagnosis but in that case, post infectious or post vaccination context is required.
As for laboratory studies, CSF examination can be helpful in excluding other diagnoses and its greatest value is demonstrating the presence of JC virus by PCR. Several studies have demonstrated its high sensitivity and specificity in PML [8].
Brain biopsy has a 100% specificity and 65 to 95% sensitivity in confirming the diagnosis. Histologically, it may present demyelination or oligodendroglia with enlarged amphophilic nuclei located at the periphery of the lesions. Another finding is “Weird” reactive astrocytes with hyperchromatic nuclei, which resemble tumor cells [9].
This disease can be life-threatening with a very poor prognosis in the absence of treatment (fatal in 3 to 4 months). Treatment with Antiretroviral Therapy (ART) may prolong survival.
References
- Hodel J, Darchis C, Outteryck O, Verclytte S, Deramecourt V, et al. Punctate pattern: A promising imaging marker for the diagnosis of natalizumab-associated PML. Neurology. 2016; 86: 1516-23.
- Berger JR. Progressive multifocal leukoencephalopathy. In: Handbook of Clinical Neurology. Elsevier; 2014; 357-76.
- Wijburg MT, Witte BI, Vennegoor A, Roosendaal SD, Sanchez E, Liu Y, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. Journal of Neurology, Neurosurgery & Psychiatry. 2016; 87: 1138-45.
- Wattjes MP, Vennegoor A, Steenwijk MD, de Vos M, Killestein J, et al. MRI pattern in asymptomatic natalizumab-associated PML. Journal of Neurology, Neurosurgery & Psychiatry. 2015; 86: 793-8.
- Sarbu N, Shih RY, Jones RV, Horkayne-Szakaly I, Oleaga L, et al. White Matter Diseases with Radiologic-Pathologic Correlation. RadioGraphics. 2016; 36: 1426-47.
- Thurnher MM, Boban J, Rieger A, Gelpi E. Susceptibility-Weighted MR Imaging Hypointense Rim in Progressive Multifocal Leukoencephalopathy: The End Point of Neuroinflammation and a Potential Outcome Predictor. AJNR Am J Neuroradiol. 2019; 40: 994-1000.
- Balashov K. Imaging of Central Nervous System Demyelinating Disorders: CONTINUUM: Lifelong Learning in Neurology. oct 2016; 22: 1613-35.
- Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, et al. PML diagnostic criteria: Consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013; 80: 1430-8.
- Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, et al. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathologica. 2005; 109: 449-55.