
Review Article
Austin J Radiol. 2023; 10(3): 1219.
A Radiological Review of the Varied Duodenal Lesions with Histopathological Correlation
Ramachandran R¹; Dhamodaran J¹*; Paneerselvam P¹; Balasubramanian S²; Rangasami R¹
¹Centre of Excellence in Radiology and Imaging Sciences, Sri Ramachandra Institute of Higher Education and Research, Chennai, India
²Department of Pathology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India
*Corresponding author: Jeevitha Dhamodaran Centre of Excellence in Radiology and Imaging Sciences, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India. Tel: 7094472904 Email: djeevithaa15@gmail.com
Received: July 03, 2023 Accepted: August 07, 2023 Published: August 14, 2023
Introduction
Majority of the duodenal lesions have received little attention in imaging literature in comparison with jejunum and ileum as most of them are primarily investigated with conventional endoscopy. Cross sectional imaging techniques–Multi Detector Computed Tomography (MDCT) and Magnetic Resonance Imaging (MRI) have dramatic improvement in diagnostic capabilities as they can analyse the wall of the duodenum, intraluminal content, submucosal extent of the disease, and infiltration into the surrounding viscera. Few of the duodenal lesions have overlapping imaging characteristics and it is crucial to correlate these conditions with histopathological analysis for definitive diagnosis. The main aim of this imaging review is to elucidate the diverse spectrum of duodenal lesions with emphasis on CT and MRI imaging features.
Anatomy
The duodenum is the first part of the small bowel and has a complex anatomicalposition and important visceral relationships which poses a challenge. The normal duodenum measures approximately 25 cm in length and 2.5 cm in transverse diameter, with mucosal folds measuring roughly 2 mm in thickness. Traditionally, the duodenum is divided into four segments [1]. The first portion, commonly known as the duodenal bulb, is intraperitoneal and extends from the gastric pylorus to the level of gallbladder neck. The second portion includes an upper and lower flexure, extends retroperitoneally from the gallbladder neck to the level of the lumbar spine. The third portion extends retroperitoneally from right to left and traverses anterior to inferior vena cava and aorta. The fourth portion ascends to the ligament of Treitz [4]. The serosa surface of the descending duodenum is closely related to the pancreatic head, forming the pancreaticoduodenal groove, an anatomic space that contains pancreaticoduodenal arterial arcades, mesenteric veins, and lymphatics. Both bile and pancreatic fluids drain into the duodenum, via ductal insertions which could have varied anatomical location.
Congenital Anamolies
Duodenal Diverticulum
The duodenum arises from the embryonic midgut and is composed of both endodermal and mesodermal tissue. Due to abnormalities in recanalization, true duodenal diverticula and duplication cyst arises [2]. The most common location of the duodenal pseudo diverticula is along the medial wall of 2nd and 3rd part of duodenum [3]. When these pseudo diverticula compress the intrapancreatic portion of common bile duct causing obstructive jaundice, it is called as lemmel syndrome [4]. These patients may sometimes present with duodenal diverticulitis, which can be complicated by perforation into the retroperitoneal space portion. On CT, diverticula appear as focal out pouching from second and third part of duodenum filled with either air, fluid or both air & fluid [3].
Duplication Cyst
Duplication cysts can occur anywhere in the alimentary tract. Approximately 12% involve the gastroduodenal region, usually along the medial aspect of the D2 or D3 segment of the duodenum [2]. They are usually asymptomatic and are often incidentally detected, but symptoms related to obstruction or secondary infection can occur. The pathognomonic imaging finding of on ultrasound includes “gut signature sign”- that shows a classical five - layered cyst [5,6]. CT shows a non-enhancing low-attenuation cystic mass with a peripheral enhancing rim. On MRCP, we could appreciate a well circumscribed T2 hyperintense lesion with a hypointense wall that shows no obvious communication with the duodenum [7].
Annular Pancreas
It is a rare congenital anomaly in which the normal pancreatic tissue encircles the second portion of duodenum either completely or incompletely. Embryologically, the pancreas develops from the dorsal (single) and ventral (bifid) pancreatic buds of primitive foregut. During normal development, one of the ventral bud (left) atrophies and the right ventral bud rotates to fuse with the dorsal bud. Persistence of the bifid ventral bud or malrotation of a portion of the right ventral bud encircles the 2nd part of duodenum resulting in annular obstruction [8]. It presents normally during childhood with features of gastrointestinal or biliary obstruction and are mostly associated with other congenital anomalies (Tracheoesophageal fistula, imperforate anus or Hirsprung disease) [9]. However, it can present in adulthood as peptic ulcer disease or pancreatitis [9]. Annular pancreas may be detected on CT as a ring of pancreatic tissue encasing the descending or 2nd part of duodenum either completely or incompletely (Figure 3.1).
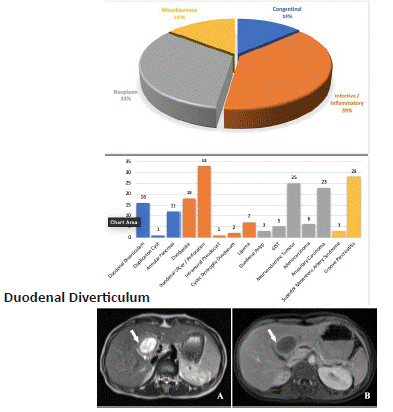
Figure 1.1: A 52 year old male with abdominal pain and vomiting for 3 days- Axial (A) and reformatted sagittal (B) view of CECT abdomen in venous phase shows a diverticulum (arrow head) arising from the medial aspect of second part of the duodenum causing upstream dilation of the CBD (left arrow) resulting in lemmel syndrome

Figure 2.1: A 40 year old male with abdominal pain for one month. Axial unenhanced T2-W (A) and post contrast axial T1-W (B) MR images in venous phase show a well-defined T2 hyperintense lesion with a hypointense wall (arrow) showing significant enhancement, which is indicative of the gut signature. The lesion is noted in the porta hepatis displacing the cystic duct and the proximal common bile duct posteriorly with no communication between the duodenum and the bile duct.
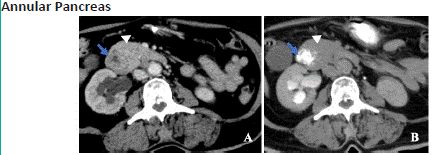
Figure 3.1: A 32 year old female with abdominal pain and post-prandial fullness for 3 weeks. Post contrast axial (A) view in venous phase and axial view with oral contrast (B) of CT abdomen shows the pancreatic parenchyma (arrow head) incompletely encircling the second part of duodenum (blue arrow) suggestive of incomplete annular pancreas.
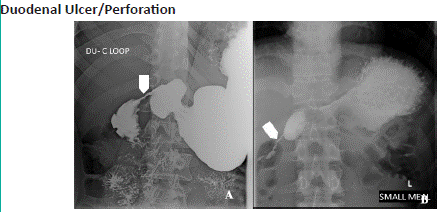
Figure 5.1: A 42 year old male with abdominal pain, nausea and vomiting. Barium meal study of Duodenal C-loop (A) and (B) shows concentric smooth narrowing in the second part of duodenum (approximately 27 mm length), with proximal dilatation of duodenal cap.

Figure 5.2: Chest X-ray AP view (A) shows air under diaphragm (right arrow) indicative of pneumoperitoneum. Axial views of CECT abdomen in venous phase shows air pockets adjacent to gastroduodenal junction(arrow) (B), multiple extra luminal air pockets outlining the thickened jejunal walls and air within the peritoneum(star) (C) and the portal venous gas (short arrows) (D), which is indicative of pneumoperitoneum. Photomicrography (E) (original magnification, 4x, haematoxylin-eosin stain) showing duodenal mucosa with ulceration. (F) (original magnification, 40x, haematoxylin-eosin stain) showing fibro collagenous tissue with focal ischaemic changes-corresponding to duodenal perforation associated with ulcer.
Infective and Inflammatory Etiology
Duodenitis
Inflammation of duodenal mucosa associated with an ulcer is more frequent and common than a duodenal ulcer alone [10]. It often co-exists with gastritis. Duodenitis can occur secondary to an infective (like H. pylori, giardiasis) or non-infective inflammatory processes (like pancreatitis, Crohn’s disease, NSAIDs, alcohol) and are typically located in the 1st and 2nd part of duodenum proximal to the ampulla of Vater [10]. On CT, the imaging features includes enhancing thickened irregular folds (>3mm), enhancing nodules or nodular folds with surrounding inflammatory changes and rarely as erosions. These erosions are superficial (as opposed to duodenal ulcer) but if present is a definite sign of duodenitis. Gastric hypersecretion which might produce fold thickening in the proximal duodenum may mimic duodenitis radiologically.
Duodenal Ulcer / Perforation
Duodenal ulcers are more commonly found in the duodenal bulb and ulcers in the post-bulbar region should raise the possibility of an underlying pathology like Zollinger-Ellison syndrome or Crohn’s disease [2,9]. UGI scopy has largely replaced fluoroscopy as the mainstay investigation of peptic ulcers. However, CT is the modality of choice in an acute setting to detect complications like obstruction, bleeding, and perforation and is done prior to endoscopy [9]. On CECT, the possible imaging findings in an ulcer include focal discontinuity in the hyper enhanced duodenal mucosa, luminal outpouching and surrounding inflammatory changes in the periduodenal space. The presence of ectopic gas, periduodenal fluid collection, and extravasation of the contrast material into the periduodenal space or lesser sac are the signs suggestive of perforation [9]. On plain radiography, the Rigler sign (free peritoneal gas outlining the bowel wall on both sides), football sign (free peritoneal gas outlining the abdominal cavity seen in case of massive pneumoperitoneum), Inverted ‘V’ sign (free gas outlining the lateral umbilical ligament),Telltale triangle sign (small radiolucent triangular air pocket seen between three bowel loops or between the flank and two bowel loops), falciform ligament sign (free gas crossing the mid-line and accentuating the falciform ligament) and sub-diaphragmatic collection of air indicates perforation [10].
Intramural Pseudocyst
Pseudocysts occurring in the wall of GIT are very rare. The most common location in GIT is the stomach and duodenum (mainly proximal to ampulla of Vater) [11]. The exact mechanism of the formation of the pseudocysts in the GIT wall is unknown. But, the possible mechanisms include rupture of a pancreatic pseudocyst into the gut wall, presence of a fistulous tract between the pancreas and the alimentary tract or inflammation of heterotopic pancreatic tissue within the gut wall [11]. CT plays a significant role in ascertaining the cystic nature of the condition and the background imaging features of acute or chronic pancreatitis, indicating the cystic lesion secondary to pancreatic pathology [11,12]. The characteristic imaging findings of intramural pseudocyst include a well-defined round or oval thin walled cystic lesion within the duodenal wall (Figure 6.1). A large intramural pseudocyst in the duodenum can cause pyloric obstruction. US or CT guided percutaneous aspiration of amylase-rich fluid confirms the diagnosis [12].
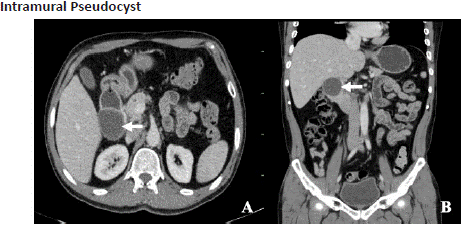
Figure 6.1: A 36 year old female with abdominal pain. Axial (A) and reformatted coronal (B) view of CECT abdomen in venous phase shows a thin walled intramural cystic lesion (left arrow) involving the posterolateral wall of 2nd part of duodenum abutting the head of pancreas. However fat plane is maintained.
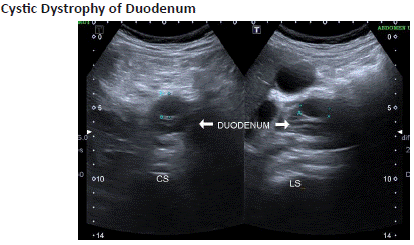
Figure 7.1: A 60 year old male with abdominal pain, vomiting. Ultrasound abdomen shows a relatively hypoechoic cystic lesion (blue arrow) along the wall of 2nd portion of the duodenum (white arrow).
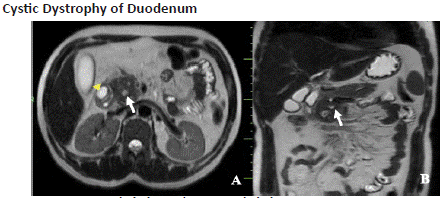
Figure 7.2: Axial (A) and coronal (B) T2-W SSFSE sequence of MR imaging shows (A)-mild circumferential wall thickening of the second part of the duodenum with a T2 hyperintense cystic lesion(arrowhead) in its anterolateral wall causing luminal narrowing, (B)- diffusely bulky pancreas, predominantly involving the head and uncinate process with peripancreatic inflammatory stranding and dilated main pancreatic duct (white arrow).

Figure 9.1: A 62 year old male presented with abdominal pain. Unenhanced axial (A) and post-contrast axial views of CT images in the arterial (B) and venous (C) phase shows a well-defined round polypoidal intraluminal soft tissue density mass (down arrow) in the medial wall of the D1 segment showing homogenous arterial enhancement with further enhancement on the venous phase causing partial narrowing of the duodenal lumen. (D) -Photomicrography-(original magnification 40x (B), haematoxylin-eosin stain) shows tubulovillous architecture with dysplastic epithelium and nuclear stratification-corresponding to tubulovillous adenoma.
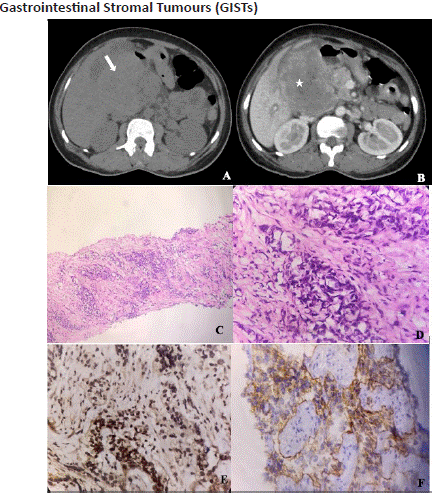
Figure 10.1: A 49 year old male with abdominal pain and Upper GI Bleed. Axial unenhanced (A) and post-contrast (B) axial views of CECT abdomen in the venous phase shows a large peripherally enhancing exo-endophytic soft tissue density lesion (white arrow) with central non-enhancing necrotic area (star) involving the antrum, D1 and D2 portions of duodenum, indicative of malignant GIST. Photomicrography-(original magnification- 4x (C) and 40x (D), haematoxylin-eosin stain) shows infiltration by the tumour cells arranged in nests. Each independent cells show nuclear atypia with vesicular to hyperchromatic nucleus with scant eosinophilic cytoplasm surrounded by desmoplastic stroma. Immunohistochemistry-(original magnification-200x, vimentin (E) and CD117 (F)) showing positivity in tumour cells indicating a case of poorly differentiated epithelioid variant of GIST.
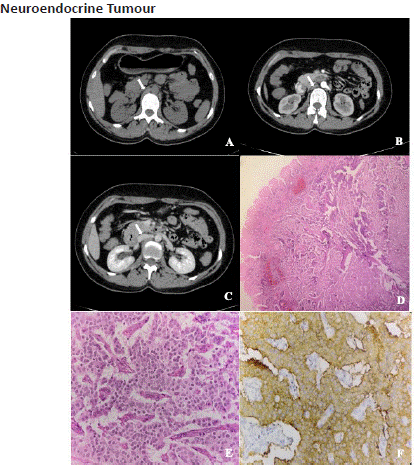
Figure 11.1: Axial unenhanced (A) and post-contrast axial views of CECT abdomen in the arterial (B) and venous (C) phase shows an intra-mural soft tissue density lesion in the D2 segment of duodenum which shows intense arterial enhancement and washout on the venous phase. Photomicrography-(original magnification- 4x (D) and 40x (E) haematoxylin-eosin stain) shows cells with pale-pink cytoplasm and rounded eccentrically placed nuclei and a strong synaptophysin positivity (F) indicating a case of well-differentiated Neuroendocrine tumour.

Figure 12.1: Axial unenhanced (A) and post-contrast axial views of CECT abdomen in the arterial (B) and venous (C) phase shows an asymmetric irregular circumferential growth (right arrow) involving the D1 portion of duodenum causing significant gastric outlet obstruction (arrowhead) with further enhancement on venous phase without washout on delayed phase (D). Enlarged peri-lesional lymph node (left arrow) was also noted. Photomicrography-(original magnification, 4x (E), haematoxylin-eosin stain) showing mucosa with adjacent poorly differentiated carcinoma. Photomicrography-(original magnification, 40x (F), haematoxylin-eosin stain) showing malignant cells.
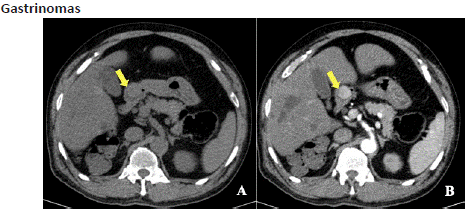
Figure 13.1: Axial unenhanced (A) and post-contrast axial (B) view of CECT abdomen in arterial phase shows a well-defined soft tissue density lesion with intense arterial enhancement at the conflux of pyloric antrum and the D1 segment of duodenum (yellow arrow).
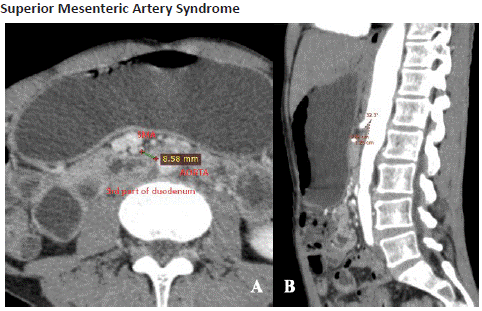
Figure 14.1: Axial (A) and reformatted sagittal (B) views of CECT abdomen in the arterial phase shows dilated D1 segment of duodenum and stomach with partial luminal narrowing of D2 segment, secondary to reduced aorto-mesenteric distance of 8. 5mm and reduced aortomesenteric angle of 32.3 degrees.
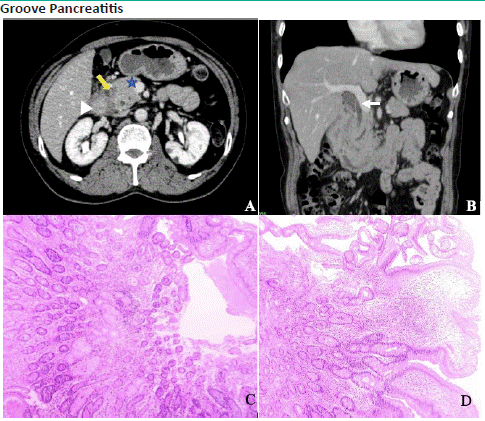
Figure 15.1: Axial (A) and reformatted coronal (B) views of CECT abdomen in the venous phase shows a sheet like infiltrative crescentic soft tissue thickening (arrow) in the pancreaticoduodenal groove which is associated with thickening of the medial wall of D2 portion of duodenum(arrow head) , inflamed and bulky appearing head of pancreas (star) and upstream dilatation of the Common bile duct (left arrow) and the pancreatic duct, which is indicative of “segmental” variant of groove pancreatitis. Photomicrography-(original magnification, 4x (C) and 40x (D), haematoxylin-eosin stain) shows marked duodenal wall thickening with Brunner gland and smooth muscle hyperplasia, oedema, and inflammation.
Cystic Dystrophy of Duodenum
Cystic dystrophy of duodenum is a sub-type of para-duodenal pancreatitis described by the presence of multiple true cysts surrounded by inflammation and fibrosis within the heterotrophic pancreatic tissue located in the duodenal wall. The second part of duodenum is most involved as it lies in close vicinity to the pancreas. It predominantly occurs in male patients with underlying chronic calcific pancreatitis [13]. On CT/ MRI, the lesion can demonstrate either the cystic or solid pattern of the disease with the associated inflammatory changes along the head of pancreas. Cystic variant shows, multiple, small, elongated or bilobed cysts (cyst size, usually >1 cm) through the thickened duodenal wall which is sometimes misdiagnosed as intramural pseudocysts. In the solid variant, microcysts (cyst size, usually <1 cm) are seen embedded within the thickened duodenal wall [11]. These inflammatory changes can eventually lead to narrowing of the duodenal lumen causing gastric outlet obstruction. Endoscopic ultrasound also plays a significant role in detection of these lesions.
Neoplasm
Duodenal Lipoma
Lipomas are benign mesenchymal tumours that contain adipose tissue. The duodenal lipomas occupy the third common site in the GI tract, following colon and ileum [15]. In most cases, lipomas are symptomless and are usually incidental finding in patients requiring imaging for other conditions. They can present either as a submucosal or an intraluminal mass. Lipomas >2 cm may cause obstruction and produce symptoms [14]. The hallmark imaging findings include a solitary, well circumscribed fat containing lesion with negative Hounsfield values (-60 to -120 HU) [14] that shows no contrast enhancement.
Duodenal Polyp
Duodenal polyps are less common compared to gastric polyps. Duodenal polyps in general are solitary and typically arise from the medial wall of 1st or 2nd part of the duodenum, expect in polyposis syndrome [16]. Most of the duodenal polyps are of adenomatous type, which are composed of dysplastic epithelium. Depending upon the predominant glandular architecture, they are classified as tubular, tubulo-villous or villous adenomas. Villous adenomas have greater risk of malignant transformation [17]. Most adenomatous polyps in the duodenum are usually benign and vary from 1 mm to 2 cm in size that rarely produces duodenal obstruction. On CT, adenomas can present as a smooth, lobulated, frond like filling defect or a well-defined, sessile, or pedunculated enhancing soft tissue density mass with smooth margins. The conclusive diagnosis of duodenal adenomas is given by histopathological examination [11]. However, the main role of CT is to detect malignant features like ulceration, lymph nodes, vascular invasion, or metastasis.
Gastrointestinal Stromal Tumours (GISTs)
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal neoplasms of the alimentary tract and account for less than 1% of GI tumours [19]. Most GISTs (60-70%) originate in stomach while 20-30% arise from the small bowel [20]. GIST arises from interstitial cells of Cajal found in the walls of the GIT, which express c-KIT (CD117) protein [18]. On CT, GISTs appear as a large, solid, exo-endophytic, heterogeneously enhancing soft tissue density lesion that typically shows circumferential rim enhancement surrounding the central low attenuation areas (which is likely due to necrosis, haemorrhage or cystic formation). CT features that may suggest malignancy include: diameter >5 cm; ill-defined margins; heterogeneous attenuation and enhancement; central necrosis; invasion of surrounding structures; hepatic metastasis and peritoneal dissemination [21]. Calcifications and Lymph node involvement are uncommon findings of GIST.
Neuroendocrine Tumour
Neuroendocrine Tumours (NET’s) account for 2% to 8% of all tumours of the gastrointestinal tract. Most of the duodenal NETs are discovered in the D1 and D2 portions of duodenum. G-cell (gastrin cell) tumours are the most prevalent form of duodenal NET (65%), and one-third of these are functional tumours (gastrinomas). D-cell (somatostatinomas) tumours are the second common type, which occurs exclusively in and around the ampulla of Vater [15]. Carcinoid tumours are a subgroup of NETs that most often affects the alimentary canal. Carcinoids in the duodenum accounts for <3% of all carcinoid tumours and are closely related to Multiple Endocrine Neoplasia type 1 (MEN-1) and Neurofibromatosis type 1 (NF-1) [15,22]. Most carcinoid tumours are non-functional and causes obstructive symptoms and rarely manifest with carcinoid syndrome.
On CT, NETs often appear as a focal polypoidal or an intra-mural mass that shows avid enhancement on early arterial phase which may or may not washout on the venous phase. Early arterial-phase enhancement is the main criteria that distinguishes NETs from other duodenal masses [15]. Nodal and distant metastases are uncommon. Carcinoid tumours occurring in ampullary region are more aggressive and presents with metastasis (more frequently to liver and lymph nodes) at the onset [23].
Duodenal Adenocarcinoma
Adenocarcinomas represent up to 80% - 90% of all primary malignant duodenal tumours with the 2nd part of duodenum being the predominant location [22]. More than 50% of these patients present with lymph node metastasis during the time of diagnosis. MDCT is the modality of choice for staging the tumour by assessing the lymph node status, the local and distant metastasis. On CT, the tumour might be recognised as a polypoidal, fungating soft tissue mass or as asymmetrical irregular thickening of the duodenal wall with ulceration, necrosis, and luminal narrowing. Owing to the presence of fibrous component, adenocarcinomas generally show slow and late heterogeneous enhancement. Adenocarcinomas occurring in the periampullary region with 2 cm of the major duodenal papillae results in proximal dilatation of the common bile duct and the pancreatic duct, which is detected on CT as “double-duct sign” [24]. Lymphovascular invasion is the powerful indicator of the prognostic and survival outcome in a patient with duodenal adenocarcinoma [25].
Gastrinomas
Gastrinomas are defined as gastrin secreting tumours that are associated with Zollinger-Ellison syndrome (ZES), more prevalent in the duodenum. ZES is characterised by elevated serum fasting gastrin levels, which are predominantly situated in the duodenum [27]. Their size ranges from less than 1 to 5 m. 60% percent of gastrinomas are malignant; so their diagnosis and localization before surgery are very important for a complete cure. Tumours greater than 1-2 cm are easily detectable with helical CT, MRI or Octreo Scan, while those smaller than 1 cm can be detected with selective or provocative angiography and intraoperative ultrasonography [26]. Endoscopic Ultrasound is the more recently established diagnostic approach which can detect digestive tract neuroendocrine tumours with higher sensitivity. On CT, well defined lesion showing enhancement on arterial phase is seen (as the tumour is hyper vascular).
Miscellaneous
Superior Mesenteric Artery Syndrome
Superior Mesenteric Artery (SMA) syndrome refers to the vascular compression of D3 segment of the duodenum between the aorta and superior mesenteric artery. The fundamental cause of SMA syndrome is due to profound loss of mesenteric fatty tissue that normally surrounds the duodenum. This results in an abnormal acute angulation of SMA and accounts for subacute or recurrent forms of obstruction [28]. On MDCT, diagnostic criteria include an acute SMA angle (aortomesenteric angle <22° and aortomesenteric distance <8–10 mm) with the proximal dilatation of the duodenum and a sharp narrowing below the plane of SMA [9].
Groove Pancreatitis
Groove pancreatitis, also known as Para-duodenal pancreatitis is a rare entity of chronic pancreatitis involving the pancreaticoduodenal groove, which is a potential space between the pancreatic head, the duodenum, and the terminal common bile duct [30]. The hallmark feature of groove pancreatitis on imaging is the inflammation in the pancreaticoduodenal groove, which may range from mild, hazy stranding to a frankly tumefactive soft tissue infiltrate (often demonstrating a ‘sheet like’ crescentic shape that is well appreciated on coronal sections). MDCT, depicts two variants of groove pancreatitis, namely the pure and segmental variants. In the ‘‘pure’’ form, the inflammatory changes are confined only to the groove with sparing of pancreatic head and the duodenum. In the ‘‘segmental’’ variant, the scar tissue forms in the groove with inflammation and thickening of the medial duodenal wall (D2 segment) and the pancreatic head [29].
Conclusion
With recent advances in cross sectional imaging, detection and characterisation of the duodenal pathologies on CT and MR imaging have become easier. CT remains the imaging modality of choice and is increasingly utilised due to its ease of availability. Even if pathological diagnosis is confirmatory, imaging does play a vital role in recognising the lesions better, providing a pathway for further management. A thorough knowledge on differential diagnosis of duodenal pathologies, will help in reducing the requisite of additional investigations and improves patient management.