
Research Article
Austin J Radiol. 2024; 11(1): 1226.
Improving PTV Coverage by Bolus: A Less Commonly Used Entity
Anu Roy1; Jitendra Nigam2; Silambarasan NS3; Navitha S3*; Piyush Kumar4
1Intern Medical Physicist, Department of Radiation Oncology, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly
2Associate Professor and Medical Physicist, Department of Radiation Oncology, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly
3Assistant Professor and Medical Physicist, Department of Radiation Oncology, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly
4Professor and Head, Department of Radiation Oncology, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly
*Corresponding author: Navitha Silambarasan Assistant Professor and Medical Physicist, Department of Radiation Oncology, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly. Tel: +919677760497 Email: navitha.selvi@gmail.com
Received: December 29, 2023 Accepted: February 03, 2024 Published: February 10, 2024
Abstract
Introduction: In external beam radiotherapy, mega voltage photon beams have a skin sparing effect. So, a bolus can give dose build-up and deliver ample doses to the superficial lesions. The aim of this study is to analyze the impact of bolus in Skin dose and PTV coverage in IMRT plans for Head and Neck malignancies.
Materials and Methods: Patients of various Head and Neck carcinomas treated with IMRT Plans were recruited for this retrospective study. Eclipse version 13.6 TPS was used for planning. All 20 patients were treated with IMRT plan without a bolus. The skin was contoured with 5 mm from the body outline. Another plan was created using a 5 mm virtual bolus linked with all the fields followed by optimization for inverse planning. Objectives of PTV and OARs were maintained constant in both the plans. Statistical analyses of both plans were performed using Student T test.
Results: There was a significant increase in the skin dose with bolus than in the non-bolus plans (P<0.00001). But on taking into consideration of patients with PTV closer to the skin, plans using bolus can give a better coverage (V95% – P=0.02). The dose received by the OARs was not statistically significant.
Conclusion: The use of bolus is an efficient method to get a better target coverage, where PTV is closer to the Skin. But bolus can increase the mean dose to the Skin. So, it is necessary to fabricate and place the bolus precisely on the patient to reduce the risk of skin toxicities.
Keywords: Bolus; Intensity Modulated Radiation Therapy; Skin dose; Superficial tumor; Virtual bolus
Abbreviations: AAA: Anisotropic Analytical Algorithm; CI: Conformity Index; CT: Computed Tomography; DICOM: Digital Imaging and Communication in Medicine; DVH: Dose Volume Histogram; EBRT: External Beam Radiation Therapy; HI: Homogeneity Index; ICRU: International Commission on Radiation Units and Measurements; IMRT: Intensity Modulated Radiation Therapy; IGRT: Image Guided Radiation Therapy; LINAC: Linear Accelerator; MV: Mega Voltage; MU: Monitor Units; OAR: Organ at Risk Volume; PTV: Planning Target Volume; PRV: Planning Organ at Risk Volume; PRO: Progressive Resolution Optimiser; TPS: Treatment Planning System; VMAT: Volumetric Modulated Arc Therapy; 3DCRT: Three Dimensional Conformal Radiation Therapy.
Introduction
Cancer is a most frequent cause of death across the world accounting for nearly one in six deaths. It is a disease in which some of the body’s cells grow uncontrollably and spread to other parts of the body [1]. Tobacco use, alcohol consumption, unhealthy diet, physical inactivity, and air pollution are risk factors for cancer and other non-communicable diseases [2] There are different types of cancer treatments that were available. In radiotherapy high energy radiation such as gamma rays, x-rays, and other sub-atomic particles are used to eradicate or manage cancerous cells or tumours. These radiation beams can be generated by 60Co machine, which produces gamma radiation or linear accelerator (also known as LINAC), producing high-energy x-rays beam. External Beam Radiation Therapy (EBRT) comes with many types depending on the beam energy, beam size and beam shape. These include conventional external beam radiotherapy, Three-Dimensional Conformal Radiation Therapy (3D-CRT), Intensity Modulated Radiation Therapy (IMRT), Proton Beam Therapy, Image Guided Radiation Therapy (IGRT), stereotactic radiation therapy, particle therapy and neutron beam therapy [3].
Head and neck cancers are common in several regions of the world. When patients with head and neck malignancies undergo EBRT, superficial gross disease is included in the target volume [4]. IMRT has been increasingly used for such cancers. When compared to traditional 3D-CRT, IMRT enhances the Planning Target Volume (PTV) coverage and effectively reduces the higher dose delivered to Organs at Risk (OARs). High energy photon beams for radiation therapy exhibit skin-sparing properties [5].
This skin sparing near the surface inside a patient is caused by a dose build-up effect of mega voltage photon beam. The absorbed dose increases within a certain depth beyond the surface until they reach a maximum before mega voltage photon beam reaches electron equilibrium [6]. The ability to spare the skin is very useful for many different types of cancer; however, there is a problem with the treatment of superficial lesions near the skin surface [7]. Thus, a build-up material, bolus is placed in direct contact with the patient's skin surface in order to increase the superficial dose and improve dose uniformity by compensating for missing tissue [8].
Bolus is a material which has properties equivalent to tissue when irradiated. Bolus material can effectively modify the radiation dose to the skin and mucosal surfaces [9]. Several types of commercially available bolus materials are often used in RT units [10].It is important in clinical practice that the bolus material is sufficiently elastic and deformable in order to conform to the surface and not adversely affected by high dose levels, be durable, non-toxic, and cost effective [11]. Bolus materials should be nearly tissue-equivalent and allow sufficient surface dose boost. In addition of bolus causes an increase in the skin dose, which may lead to increased risk of skin toxicities like radiodermatitis which can decrease patients' overall quality of life [12].
Aim of the Study
This study aimed to evaluate the Skin dose and PTV coverage in IMRT plans with and without the bolus for head and neck malignancies and to analyse the effect of bolus in the dosimetric indices and other treatment parameters like monitor units and treatment time.
Materials and Methods
Patient Selection
For this retrospective study, twenty patient plans were randomly selected from the list of patients who had received IMRT for head and neck cancer. The patient plans were developed using Varian Medical systems Treatment Planning Systems (TPS), Eclipse of version 13.6. All patients were immobilized with Klarity five push pin head and neck thermoplastic cast in the Head and Neck base frame. Patients were positioned in supine position with their arms alongside their body. All the CT scans were taken using contrast which is used differentiate tumor volume from others. All of the CT dataset were acquired using a Siemens Somato Scope CT 32 Slice scanner. The CT image was taken at 3 mm slice thickness. These images were taken from supra orbital to trachea bifurcation. The data were transferred to the TPS using DICOM format.
Delineation of Structures
Targets and OARs were contoured in the 3mm CT slices by a Radiation Oncologist. To analyze the dose received by the skin, it was contoured with 5 mm from the body outline. The 5 mm thickness was chosen to include three layers of the skin (epidermis, dermis, and hypodermis) as per Timmerman guidelines [13].
Treatment Planning
All plans were developed using the Eclipse version 13.6 TPS. The treated IMRT plan consists of 7 or 9 beams (6 MV) around the PTV to get a optimal dose distribution. Figure.1 gives a pictorial representation of the beam orientation used for the planning. A new IMRT plan was created using same beam orientations and energy. A 5 mm virtual bolus was designed during treatment planning which is specifically tailored to overlay only superficial regions of the PTV, thus sparing dose build up to normal skin and was linked with all the fields followed by Progressive Resolution Optimization (PRO) for inverse planning. This virtual bolus must then be fabricated and positioned before patient treatment The transverse view of CT slice of a patient which shows the bolus, skin contour and the PTV is given the Figure II. The Objectives of PTV and OARs are maintained constant in the plans with and without a bolus. The 3D dose was calculated using Anisotropic Analytical Algorithm (AAA) with 2.5mm dose calculation grid size. MU’s (Monitor Units) were obtained for each of the fields after the dose calculation.
Figure 1: Beam orientations used for the IMRT planning.
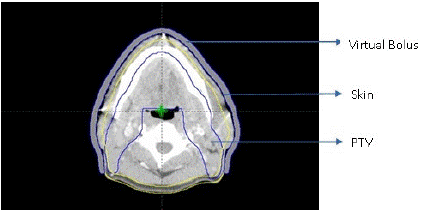
Figure 2: Transverse view of CT slice with virtual bolus, skin contour and PTV.
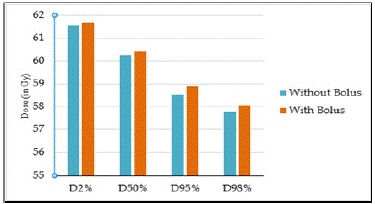
Figure 3: Comparison of D2%, D50%, D95% and D98% in the plans with and without the bolus.
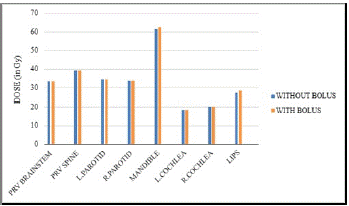
Figure 4: Comparison of OAR doses for plans with and without a bolus.
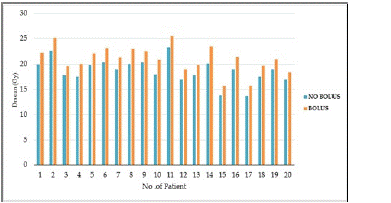
Figure 5: Comparison of skin dose in plans with and without the bolus.
Dosimetric Analysis
The coverage of the PTV was measured in this study by comparing the maximum, median and mean doses received by the PTV. The plans were evaluated using dose statistics and Dose Volume Histogram (DVH) [14].
The following OAR parameters were estimated from the DVH like maximum dose (Dmax) for PRV Brainstem, PRV Spine and Mandible. Mean dose (Dmean) received by parotid, cochlea and lips. The mean skin dose was evaluated from the dose statistics for both the plans. Monitor units and treatment time is calculated for both sets of plans for the entire patient. Statistical analyses of both plans were performed for PTV, skin and OARs using a Student’s T test.
Results
PTV Coverage and Dose Distribution
For all IMRT plans, the ICRU dose prescription protocol was followed, with a minimum coverage dose of 95% and a maximum hot spot dose of 107 % of the prescribed dose to the PTV. The dose reporting was also done as per ICRU level 2 reporting.
From the above table and graph it is evident that the plans with the bolus give a higher dose to the PTV than the plans without a bolus. The Dmean, D50%, D98% and D95% values are highly significant and this shows the advantage of using a bolus for superficial lesions.
OAR Doses
The OAR doses were not much significant in all the patients. In both the plans it was able to achieve the constraints for all the OARs.
Skin Dose
The mean dose received to skin was highly significant in the plans which use a bolus. This can be seen evident in the following figure.
Monitor Units and Treatment Time
From the results the MUs and treatment time was quite higher in the plans without a bolus than in the plans with bolus. The mean MUs and treatment times for all the patients are given in the Table 3.
Without Bolus (Gy)
With Bolus (Gy)
P Value
Dmean
60.10 ± 0.31
60.33 ± 0.42
0.0181
D50%
60.23 ± 0.32
60.41 ± 0.41
0.0408
D98%
57.75 ± 0.71
58.06 ± 0.52
0.0188
D95%
58.52 ± 0.47
58.89 ± 0.41
0.0019
D2%
61.56 ± 0.39
61.68 ± 0.53
0.1769
Table 1: PTV Coverage.
Without bolus (Gy)
With Bolus (Gy)
P value
PRV Brainstem (Dmax)
33.323±8.00
33.321±8.05
0.4966
PRV Spine (Dmax)
39.183±5.93
39.355±6.10
0.1803
Mandible (Dmax)
61.523±4.54
62.269±2.61
0.2491
Parotid-Right (Dmean)
33.823±10.89
33.857±11.06
0.3885
Parotid-Left (Dmean)
34.527±13.02
34.584±13.01
0.3047
Cochlea-Right (Dmean)
20.263±10.17
20.026±10.05
0.0316
Cochlea-Left (Dmean)
18.365±11.36
18.302±11.62
0.4067
Lips (Dmean)
27.356±8.26
28.226±8.45
0.0033
Table 2: Dose to OARs.
Without Bolus
With Bolus
P Value
MU
1466
1385
0.0030
Treatment time (Min)
3.67
3.46
0.0030
Table 3: MUs and Treatment time.
It can be seen that the plans with a bolus gives a reduced number of MUs and treatment time which in turn can help the reduction of patients on couch time.
Discussion
Although there has been development in treatment techniques, such as 2D to 3D-CRT, IMRT, and VMAT, radiotherapy has long been utilised to treat head and neck cancer. It has also been treated in a variety of ways depending on the stage of the cancer.
In this study in most of the patient’s virtual bolus improved the volume receiving the reference isodose of the prescribed dose to the PTV by a clinically significant amount (p=0.02). Similarly in a study conducted by Shenoy et.al, [15] it was possible to achieve clinically acceptable coverage for all plans for all patient when using a bolus. In fact, it was possible to cover 97% of the PTV with the 95% isodose in all cases.
In a study by Andrew Luu et.al, [16] the virtual bolus improved the minimum dose to the superficial CTV. The improved dose distribution in the plans with bolus is due to fact that bolus acts as dose build up and deliver maximum dose to the target which includes superficially located positive GTV nodes. But there was not much difference in the conformity and homogeneity of the prescribed dose to the target.
Tyran et.al, [17] conducted a study about benefit of using a virtual bolus during treatment planning for breast cancer and from their results it is seen that the VMAT plans which used a virtual bolus achieved delivery of 95% of the prescribed dose to 95% of the CTVs.
All the constraints for the OARs were achieved in both the plans. This is because the TPS will run the inverse planning optimization in order to achieve the all the given dose constraints. There were not much significant differences in the OAR doses in all the patients except lips and right cochlea. Since lip is a superficial organ and involvement of bolus over the lips can be a reason for its increased mean dose. The significance of cochlea dose can be contributed to the patient selection for the study, in which some cases are ipsilateral targets.
A study was conducted by Gina Wong et.al, [18] titled “Quantitative Effect of Bolus on Skin Dose in Post mastectomy Radiation Therapy “within a depth of 3 mm, bolus plans had a maximum skin dose 7% ± 2.5% higher than the non-bolus plans (P<.00001). Mean skin dose within depths of 3 and 5 mm were both significantly higher (P<.00001) for bolus plans.
Another study by Andic et.al, [19] “Evaluation of skin dose associated with different frequencies of bolus applications in post-mastectomy three-dimensional conformal radiotherapy” showed that the mean, minimum and maximum skin doses were significantly increased with increasing days of bolus applications (p<0.001). Bolus use in all fractions provided a 20.8%±2.8% minimum skin dose increment.
The results of this study too showed a similar pattern of skin dose increment in the plans with bolus. The difference in the mean skin dose in Gy was highly significant in the plans with bolus (p<0.0001). The high skin dose in the plans which uses a bolus can be contributed to the compensation of skin sparing of photons by a tissue equivalent bolus.
A Monitor Unit (MU) is a measure of machine output from a clinical accelerator for radiation therapy such as a linear accelerator and it represents the treatment time for a patient. The plans without a bolus give a larger number of MUs and higher value of treatment time in minutes. Since the target in most of the patients where superficial in nature and it lies in the dose build-up region of the 6 MV photon beams, TPS runs multiple fluence optimizations in order to get a prescribed dose distribution over the PTV which in turn results in the increased number of MUs.
But in case of plans with bolus the build-up is provided by a bolus hence the Dmax can be delivered to the target. The use of bolus is a better option in the treatment planning when target is superficial or when target is not getting adequate coverage. But the risk of skin toxicities is always a concern. So, more attention is required during the process of creation and positioning the bolus precisely over the required regions of the target to get a acceptable clinical result along with a balancing normal skin sparing.
Conclusion
In this study, IMRT technique was used for head and neck planning and surface doses in the original plans without bolus were compared with newly developed bolus plans. The results suggest that the use of bolus is an efficient method to achieve clinically significant improvement in target coverage, particularly when the PTV is closer to the skin. The plans with bolus, the clinical objectives were achieved like to get optimal PTV coverage, doses to OARs within their tolerance limit, maximum dose in the overall plan less than 110%.
However, the use of bolus can increase the mean dose to the skin. Therefore, the design and placement of radiation bolus material precisely is necessary to reduce the risk of skin toxicities.
Author Statements
Competing Interests
The authors have declared that no competing interest exists.
References
- National Cancer Institute at the National Institutes of Health. What is cancer?.
- World Health Organization. Cancer.
- Lawrence TS, Ten HRK, Giaccia A. Príncipe of radiation oncology. 8th ed. Philadelphia: Lippincott Williams & Wilkins Press. 2008.
- Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008; 371: 1695-709.
- Mihaylov IB, Penagaricano J, Moros EG. Quantification of the skin sparing effect achievable with high energy photon beams when carbon fiber tables are used. Radiother Oncol. 2009; 93: 147-52.
- Turner JY, Zeniou A, Williams A, Jyothirmayi R. Technique and outcome of post-mastectomy adjuvant chest wall radiotherapy-the role of tissue-equivalent bolus in reducing risk of local recurrence. Br J Radiol. 2016; 89: 20160060.
- Hsu SH, Roberson PL, Chen Y, Marsh RB, Pierce LJ, Moran JM. Assessment of skin dose for breast chest wall radiotherapy as a function of bolus material. Phys Med Biol. 2008; 53: 2593-606.
- Khan FM. The physics of radiation therapy. 4th ed. Philadelphia: Lippincott Williams & Wilkins. 2010.
- Kong M, Holloway L. An investigation of central axis depth dose distribution perturbation due to an air gap between patient and bolus for electron beams. Australas Phys Eng Sci Med. 2007; 30: 111-9.
- Chiu-Tsao ST, Chan MF. Photon beam dosimetry in the superficial buildup region using radiochromic EBT film stack. Med Phys. 2009; 36: 2074-83.
- Kudchadker RJ, Antolak JA, Morrison WH, Wong PF, Hogstrom KR. Utilization of custom electron bolus in head and neck radiotherapy. J Appl Clin Med Phys. 2003; 4: 321-33.
- Liu X, Wang Y, Guo Qishuai, Luo Huanli, Luo Q, Li Q, et al. Clinical impact of the bolus in intensity-modulated radiotherapy and volumetric-modulated arc therapy for Stage I-II nasal natural killer/T-Cell Lymphoma. Oncol Res Treat. 2020; 43: 140-5.
- Timmerman R. A story of hypofractionation and the table on the wall. Int J Radiat Oncol Biol Phys. 2022; 112: 4-21.
- The International Commission on Radiation Units and Measurements. Prescribing, recording and reporting photon-beam IMRT. J ICRU. 2010; 10: NP.2-NP.
- Shenoy. To investigate the effect of bolus on skin dose in VMAT/IMRT for head and neck cancer. 2016.
- Luu A, Doerwald-Munoz L, Ostapiak O. An evaluation of two approaches to skin bolus design for patients receiving radiotherapy for head and neck cancers. J Med Imaging Radiat Sci Sep. 2015; 46: S37-42.
- Tyran M, Tallet A, Resbeut M, Ferre M, Favrel V, Fau P, et al. Safety and benefit of using a virtual bolus during treatment planning for breast cancer treated with arc therapy. J Appl Clin Med Phys. 2018; 19: 463-72.
- Wong G, Lam E, Bosnic S, Karam I, Drost L, Yee C, et al. Quantitative Effect of Bolus on Skin Dose in Post mastectomy radiation therapy. J Med Imaging Radiat Sci. 2020; 51: 462-9.
- Andic F, Ors Y, Davutoglu R, Baz Cifci S, Ispir EB, Erturk ME. Evaluation of skin dose associated with different frequencies of bolus applications in post-mastectomy three-dimensional conformal radiotherapy. J Exp Clin Cancer Res. 2009;28(1):41. doi: 10.1186/1756-9966-28-41, PMID 19317895.