
Case Report
Austin J Radiol 2024; 11(2): 1231.
Double Metachronous Pituitary Microadenoma: Literature Review and Case Report
Soliman Alaqeel; Doaa Alzaher*
Department of Diagnostic Radiology, Dammam Medical Complex, Dammam, Saudi Arabia
*Corresponding author: Doaa Alzaher Department of Diagnostic Radiology, Dammam Medical Complex, Dammam, Saudi Arabia. Email: dr.doaalh@gmail.com
Received: April 08, 2024 Accepted: May 03, 2024 Published: May 10, 2024
Abstract
Pituitary adenomas, which are benign, slow-growing neoplasms, are commonly associated with hormonal overproduction. They rank as the third most prevalent intracranial tumor, following gliomas and meningiomas. Although double pituitary adenomas are infrequent, they manifest when two adenomas develop within a single pituitary gland. These dual adenomas exhibit typical immunohistochemical and histopathological features. They can be further classified into two distinct types: contiguous and clearly distinct. Neuroradiological imaging facilitates the identification of clearly distinct tumors. We present imaging findings from a rare case of a double pituitary microadenoma. Early diagnosis of this uncommon condition is crucial to prevent potential complications.
Introduction
Pituitary adenomas are primarily benign tumors originating from the anterior pituitary gland. They rank as the third most common intracranial neoplasms, following gliomas and meningiomas. These adenomas account for approximately 15% of all intracranial tumors and have a population prevalence of approximately 80 per 100,000 individuals. Most pituitary adenomas are monoclonal and correspond to specific pituitary cell types. However, up to one-third of these tumors express more than one pituitary hormone, leading to their designation as plurihormonal adenomas [1]. These plurihormonal adenomas can be further categorized into two types. The first type is monomorphous adenomas which consists of a single cell type capable of producing two or more hormones. The second type is plurimorphous adenomas which is composed of two or more cell types, each producing different hormones [2].
While synchronous occurrences of true double or multiple pituitary adenomas are rare, they do exist. Morphologically, these adenomas fall into two subtypes. The first subtype is clearly distinct tumors which are recognizable through neuroradiological imaging or intraoperative assessment. The second subtype is contiguous tumors which are surgically resected as a single mass, they are later confirmed as multiple pituitary adenomas upon histopathological examination [2].
The presence of multiple pituitary adenomas increases the risk of surgical failure in treating anterior pituitary gland syndromes. In some cases, hormonally active lesions may be inadvertently left behind during surgical exploration. Additionally, there are instances where multiple pituitary adenomas appear as recurrences of previously resected adenomas, each exhibiting a distinct immunohistochemical profile. Early diagnosis and appropriate management are crucial to prevent potential complications associated with these rare conditions [3].
Case Presentation
A 35-year-old female presented to the endocrinology clinic with a complaint of lack of menses for eight months. She also suffered from moderate headache which was tolerated with oral analgesic medication. She had not experienced hot flushes or other symptoms. On physical examination she has bilateral galactorrhea along with hyperpigmentation of the areola. The visual field was not impaired. Bone mineral density was normal. Hormonal testing showed hyperprolactinemia accompanied by hypogonadotropic hypogonadism.
MRI of the pituitary gland demonstrated a small pituitary lesion with typical characteristics of microadenoma (Figure 1 & 2). The patient was then started on oral Cabergoline and her hormonal profile was normalized for two years. On her follow up visit to the endocrinology clinic, the patient complained of disturbance in her menstrual cycle along with recurrent galactorrhea. Prolactin (PRL) concentration was 1439 ng/ml. The endocrinologist requested MRI of the pituitary gland to assess the status of pituitary microadenoma. Follow up MRI pituitary gland (Figure 3 & 4) demonstrated size regression of the right pituitary microadenoma with newly seen microadenoma on the left side.
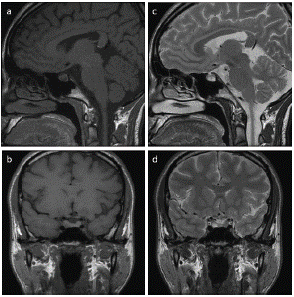
Figure 1: Sagittal (a) and coronal (b) T1 weighted image, sagittal (c) and coronal (d) T2 weighted image. Right pituitary microadenoma of low T1 and high T2 signal intensity.
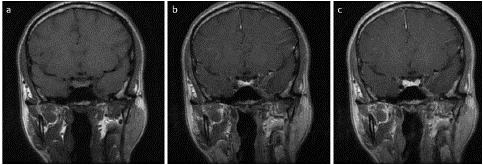
Figure 2: Coronal T1 dynamic post-contrast images, early (a), intermediate (b) and delayed (c) images. Delayed enhancement of the right pituitary microadenoma relative to the pituitary gland.
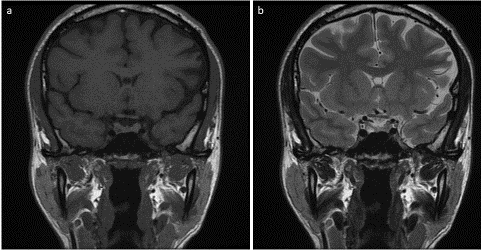
Figure 3: Coronal T1 (a) and T2 (b) weighted image. The right pituitary microadenoma regressed in size. Newly developed left pituitary microadenoma of isointense signal in T1 and T2 weighted images.
The patient was counseled for the necessity of surgical intervention. However, she refused any further intervention and preferred to seek medical advice in her hometown.
Discussion
Pituitary adenomas are benign tumors on the pituitary gland. They are typically solitary and histologically uniform. Although double pituitary adenomas are rare, they occur at an incidence rate of 0.9% in random pituitary autopsy samples. The prevalence of these tumors ranges from 0.25% to 2.6% among postoperated pituitary adenoma specimens. Multiple pituitary adenomas are characterized by the presence of two distinct histological tumors, which can either be contiguous or non-contiguous [1]. While older surgical series reported a negligible proportion of cases with multiple adenomas (12 out of 3,000; 0.4%), later studies found a higher prevalence (2.6%) among 117 unselected operated adenomas [4]. Notably, a series of 660 patients operated for Cushing’s disease revealed a prevalence of 3.3% for multiple adenomas, defined as having distinct pseudocapsules (non-contiguous). Advances in high-field MRI technology have facilitated the preoperative detection of double pituitary adenomas, aided by cytological analysis [5].
Several theories have been proposed to elucidate the pathogenesis of double pituitary adenomas. The initial hypothesis posits an unplanned monoclonal extension involving two distinct hereditarily altered types of pituitary cells, supported by specific tumor features observed during meticulous microdissection. An alternative theory, known as the transdifferentiation hypothesis, relies on the ability of pituitary adenoma cells to transform or transdifferentiate into different cell types. This concept is further substantiated through the examination of transcription factor expression and genetic profiling in double pituitary adenomas. Additionally, pituitary adenomas are categorized based on size: those measuring = 10 mm are considered microadenomas, while those exceeding 10 mm fall into the macroadenoma category [3]. Notably, Sumida et al. described pituitary adenomas observed on MRI as isointense on T1-weighted images, showing no enhancement after contrast imaging [6]. Furthermore, a study by Oner et al. identified two non-enhancing foci within the gland, separated by normal gland tissue [7]. Similarly, Zielinski et al. reported preoperative imaging revealing two microadenomas separated by normal pituitary tissue. Immunohistochemistry performed postoperatively confirmed the presence of two distinct adenoma types [8]. Overall, preoperative MRI imaging plays a crucial role in identifying dual adenomas, potentially preventing relapse and surgical complications.
MRI imaging plays a crucial role in identifying multiple separate pituitary adenomas. Precise preoperative localization of pituitary microadenomas significantly enhances surgical effectiveness, particularly in cases of Cushing’s disease. However, due to the small size of some tumors, achieving accurate localization can be challenging. State-of-the-art, high-field MRI scanners provide heightened sensitivity for detecting pituitary tumors. Additionally, specialized imaging protocols, such as dynamic and/or SPGR techniques, improve the detection of minute pituitary adenomas, although their specificity remains limited. In specific scenarios, bilateral inferior petrosal sinus sampling (BIPSS) serves as a preferred diagnostic method for pituitary-dependent hypercortisolemia and the localization of corticotroph pituitary tumors. Metionine-PET/MR and intraoperative ultrasound of the pituitary gland may offer supplementary information for localizing complex cases. Comprehensive preoperative endocrine assessment is also essential, as it may reveal the co-occurrence of multiple adenomas [9].
Detecting clearly separated multiple pituitary macroadenomas using MRI imaging appears straightforward due to their distinct signal intensities on T1 and T2 weighted MR images. However, challenges arise from the limited diagnostic capability for microadenomas. MR imaging can identify microadenomas measuring 2–3 mm with a sensitivity of only 85%. Recognizing contiguous double adenomas is more complex and relies on accurate interpretation of immunohistochemical localization of different pituitary hormones within distinct adenomatous cells, independent of endocrinological and neuroradiological assessments. In certain cases, electron microscopy plays a crucial role in precisely determining the cell type constituting a tumor and establishing the diagnosis of multiple pituitary adenomas. Recently, pituitary transcription factors (such as Pit-1, Tpit, and SF-1) have gained prominence for accurately characterizing adenoma cell types and distinguishing separate primary lesions, including collision tumors or divergent differentiation from a single lesion. Both approaches are valuable for distinguishing multiple monoclonal adenomas from single plurihormonal adenomas [10].
Conclusion
Pituitary microadenomas, although uncommon, pose diagnostic challenges due to their small size and diverse clinical presentations. These benign tumors, measuring less than 10 mm in diameter, are typically asymptomatic in early stages. However, when hormonal imbalances occur, further investigation is crucial. Early diagnosis is essential, as microadenomas can lead to vision disturbance resulting from optic chiasma compression or severe headache due to cavernous sinus compression. MRI imaging, coupled with hormonal assessment, facilitates timely identification.
References
- Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Multiple adenomas of the human pituitary. A retrospective autopsy study with clinical implications. J Neurosurg. 1991; 74: 243-247.
- Roberts S, Borges MT, Lillehei KO, Kleinschmidt-DeMasters BK. Double separate versus contiguous pituitary adenomas: MRI features and endocrinological follow up. Pituitary. 2016; 19: 472-81.
- Magri F, Villa C, Locatelli D, Scagnelli P, Lagonigro MD, Morbini P, et al. Prevalence of double pituitary adenomas in a surgical series: Clinical, histological and genetic features. J Endocrinol Invest. 2010; 33: 325331.
- Kontogeorgos G, Scheithauer BW, Horvath E, Kovacs K, Lioyd RV, Smyth HS, et al. Double adenomas of the pituitary: a clinicopathological study of 11 tumors. Neurosurgery. 1992; 31: 840-9.
- Ratliff JK, Oldfield EH. Multiple pituitary adenomas in Cushing’s disease. J Neurosurg. 2000; 93: 753-761.
- Sumida M, Uozumi T, Mukada K, Arita K, Kurisu K, Yano T, et al. MRI of pituitary adenomas: the position of the normal pituitary gland. Neuroradiology. 1994, 36: 295-7.
- Oner AY, Tokgoz N, Erbas G, Tali ET. Magnetic resonance imaging findings of simultaneous double pituitary adenoma: a case report and review of the literature. Rivista di Neuroradiologia. 2004; 17: 113-5.
- Zielinski G, Sajjad EA, Maksymowicz M, Pekul M, Koziarski A. Double pituitary adenomas in a large surgical series. Pituitary. 2019; 22: 620-32.
- Ogando-Rivas E, Alalade AF, Boatey J, Schwartz TH. Double pituitary adenomas are most commonly associated with GH- and ACTH-secreting tumors: systematic review of the literature. Pituitary. 2017; 20: 702–708.
- Mete O, Alshaikh OM, Cintosun A, Ezzat S, Asa SL. Synchronous multiple pituitary neuroendocrine tumors of different cell lineages. Endocr Pathol. 2018; 29: 332–338.