
Research Article
Austin J Radiol. 2024; 11(3): 1239.
Radiomics Approach for Predicting Esophageal Cancer Pathological Stages Based on MRI
Rihui Yang1#; Ting Dong2#; Tianhui Zhang1; Weixiong Fan1; Haodong Qin3; Guihua Jiang2*; Haiyang Dai4*
1Department of Radiology, Meizhou People’s Hospital, PR China
2Department of Medical Imaging, Guangdong Second Province General Hospital, PR China
3Siemens Healthineers, Guangzhou, PR China
4Department of Medical Imaging, Huizhou Municipal Central Hospital, PR China
*Corresponding author: Haiyang Dai, Department of Medical Imaging, Huizhou Municipal Central Hospital, No. 41, North Eling Road, Huizhou 516001, PR China; Guihua Jiang, Department of Medical Imaging, Guangdong Second Province General Hospital, Guangzhou, 510317, China. Email: d.ocean@163.com; jianggh@gd2h.org.cn
#These authors have been equally contributed to this article.
Received: July 17, 2024 Accepted: August 14, 2024 Published: August 21, 2024
Abstract
Objective: To explore a rational radiomic approach for predicting preoperative staging and lymph node metastasis based on Magnetic Resonance (MR) images in esophageal cancer patients.
Materials and Methods: This retrospective study included 120 patients (primary cohort: n= 84; validation cohort: n= 36) with esophageal carcinoma confirmed by surgery and pathology. All patients underwent a preoperative MR scan from the neck to the abdomen. For each patient, high throughput and quantitative radiomics features were extracted from T2WI and contrast-enhanced T1WI (T1WI_Gd) images. The radiomics signature of the T2WI and T1WI_Gd images was constructed using minimal redundancy maximal relevance (mRMR) and the least absolute shrinkage and selection operator (Lasso). In addition, associations between the radiomics signature and esophageal cancer staging and lymph node metastasis were explored. Finally, the diagnostic performance of the radiomics approach and tumor volume for discriminating between stages I - II and III - IV and predicting lymph node metastasis was evaluated and compared using the area under the receiver operating characteristic curve (AUC), Sensitivity (SEN), and Specificity (SPE).
Results: A total of 1316 radiomics features were extracted. After feature dimension reduction, eight and nine features were selected for the respective cohorts to build radiomics signatures. Then, based on these signatures, a logistic regression model was built to predict the stages and lymph node metastasis of esophageal cancer. The radiomics signature of T2WI with T1WI_Gd discriminated better the stages and lymph node metastasis in the primary (AUC: 0.884, 0.858; SEN: 0.953, 0.744; SPE: 0.732, 0.829) and validation cohorts (AUC: 0.765, 0.711; SEN: 0.842, 0.952; SPE: 0.647, 0.533).
Conclusions: The MRI-based radiomics signature could identify esophageal cancer stages and lymph node metastasis before treatment.
Keywords: Esophageal cancer; Tumor staging; Lymph node metastasis; Multimodal MRI
Introduction
Esophageal Cancer (EC) is one of the most common malignant gastrointestinal tumors in China, ranking sixth in malignant tumor incidence and fourth in mortality [1], with a five-year survival rate of 19% [2]. Early (stage I - II) esophageal cancer is primarily treated with minimally invasive endoscopic resection or radical surgical resection, while advanced (stage III - IV) esophageal cancer, which has a poor prognosis, is managed with surgical resection after neoadjuvant chemoradiotherapy, or chemoradiotherapy alone [3]. Notably, Lymph Node (LN) involvement is generally associated with worse overall survival [4]. Therefore, predicting EC stages and LN metastasis in patients before treatment is clinically essential. Radiomics is gaining importance in cancer research [5]. High-throughput mining extracts quantitative image features from digitally encrypted medical images, and this is coupled with robust image-based signatures that could potentially enhance precision diagnosis and treatment. Radiomics research recently revealed MRI’s potential to substantially improve the ability to detect or predict LN metastases [4,5]. However, there are no reports on whether a radiomics approach could predict the EC stage based on multimodal MRI.
Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography-CT (PET-CT), and Endoscopic Ultrasonography (EUS) have their respective advantages and disadvantages for evaluating EC preoperative stages and LN status; notably, their sensitivities and specificities are different [6-8]. Currently, the CT-enhanced scan is the most commonly used imaging method for EC diagnosis and efficacy evaluation. However, the detection accuracy of positive LN on preoperative CT remains controversial, and the reported sensitivity, specificity, and accuracy was 37.3-67.2%, 63.9-96.4%, and 85.8-87.2%, respectively [9]. Moreover, the latest version of the Chinese guidelines for EC radiation therapy proposed that the diagnostic value of MRI for LN metastasis was similar to or better than enhanced CT [10].
Relevant studies have shown that radiomics has potential value in predicting tumor stages or LN metastasis [11-13]. However to date, few studies have reported on building models based on MRI radiomics features to predict EC stages and LN metastasis. In this study, the preoperative T2WI and contrast-enhanced T1WI (T1WI_Gd) sequence radiomics features were extracted. We aimed to construct a model to evaluate their value in predicting EC stages and LN metastasis to provide a reference for the clinical treatment of patients.
Result
Clinical Data
The clinicopathologic characteristics of the patients are presented in Table 1. There were no significant differences in age, sex, tumor location, or pathological type between the two groups of patients at EC stages I - II and III – IV, or between the two groups of EC patients with positive and negative LN metastasis (P > 0.05).
Radiomic Features
We used the variance method (excluding variance as 0), mRMR method (select the top 50), and LASSO method (lambda selection standard λ.min) for dimensionality reduction. As a result, eight and nine of 1316 radiomic features were included for EC staging and LN metastasis prediction, respectively. For EC staging, these included logarithm_glcm_Maximum Probability, logarithm_firstorder_Interquartile Range, exponential_glszm_Gray Level Non-Uniformity Normalized, wavelet. HHL_glszm_Size Zone Non-Uniformity Normalized, gradient_glszm_Low Gray Level Zone Emphasis, logarithm_firstorder_Skewness, logarithm_ngtdm_Contrast, logarithm_glcm_Idn. For LN metastasis prediction, these were the included wavelet. LLH_glcm_MCC, wavelet. HHL_glcm_MCC, logarithm_glcm_Maximum Probability, wavelet. HHL_glcm_Imc2, exponential_glszm_Gray Level Non-Uniformity Normalize, gradient_glszm_Low Gray Level Zone Emphasis, logarithm_ngtdm_Contrast, gradient_gldm_Dependence Non-Uniformity Normalized, and logarithm_gldm_Dependence Variance.
The names and descriptions of the selected features are listed in Table 2. Distribution of eight and nine features that could distinguish between stages I - II and III - IV, and positive and negative LN metastasis, were analyzed using the Χ2-test, respectively. Of these, the feature “wavelet.HHL_glcm_Imc2” had a P value < 0.05.
Task
MRI
Feature Name
OR (95%CI)
P Value
TNM stages prediction
T2WI
logarithm_glcm_MaximumProbability
1.32(0.64~1.86)
0.251
logarithm_firstorder_InterquartileRange
0.86(0.39~1.36)
0.054
T1WI-Gd
exponential_glszm_GrayLevelNonUniformityNormalized
1.16(0.84~2.54)
0.654
wavelet.HHL_glszm_SizeZoneNonUniformityNormalized
0.63(0.74~0.86)
0.075
gradient_glszm_LowGrayLevelZoneEmphasis
0.95(0.55~1.34)
0.274
logarithm_firstorder_Skewness
0.76(0.63~2.16)
0.064
logarithm_ngtdm_Contrast
1.75(0.81~3.68)
0.312
logarithm_glcm_Idn
0.72(0.33~0.93)
0.476
LN status prediction
T2WI
wavelet.LLH_glcm_MCC
0.92(0.55~1.53)
0.765
wavelet.HHL_glcm_MCC
0.55(0.19~1.44)
0.231
logarithm_glcm_MaximumProbability
1.52(0.84~2.86)
0.175
0.5(0.24~0.93)
0.045*
T1WI-Gd
exponential_glszm_GrayLevelNonUniformityNormalize
0.96(0.54~1.66)
0.879
gradient_glszm_LowGrayLevelZoneEmphasis
0.71(0.26~1.76)
0.474
logarithm_ngtdm_Contrast
1.35(0.61~3.07)
0.467
gradient_gldm_DependenceNonUniformityNormalized
0.56(0.29~1.06)
0.084
logarithm_gldm_DependenceVariance
0.62(0.25~1.43)
0.276
Table 2: Selected features with descriptions.
Radiomic Signature Discrimination
The radiomic signature was built by employing the selected features in the previous section, forming a linear combination of the logistic regression model of these features. The radiomic signature’s discriminative power for TNM staging and LN metastasis was assessed by two ROCs in the primary and validation cohorts (Figure 2), respectively. Then, the model’s ROCs were tested by DeLong, and the AUC, SEN, and SPE were calculated (Table 3). The radiomic scores (Rad scores) of the EC patients in the primary and validation cohorts were calculated through the elastic net model. Each patient’s Rad scores in both the primary and validation cohorts for two tasks.
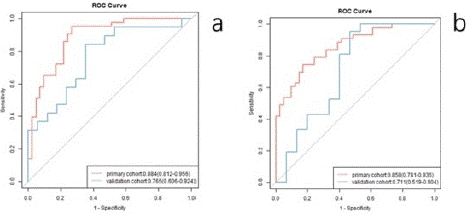
Figure 2: ROCs were employed to assess the MRI radiomic signature discriminative performance of the TNM stages and the LN metastasis in preoperative EC patients.
Task
Primary cohort
Validation cohorts
AUC (95% CI)
SEN (95% CI)
SPE (95% CI)
AUC (95% CI)
SEN (95% CI)
SPE (95% CI)
TNM stages prediction
0.884 (0.812-0.956)
0.95.3 (0.891-1.000)
0.73.2 (0.596-0.867)
0.765 (0.606-0.924)
0.842 (0.678-1.000)
0.647 (0.420-0.874)
LN status prediction
0.858 (0.781-0.935)
0.744 (0.614-0.875)
0.829 (0.714-0.944)
0.711 (0.519-0.904)
0.952 (0.861-1.000)
0.533 (0.281-0.786)
Table 3: Laboratory values at hospital admission.
Discussion
This study showed that a quantitative approach based on multimodal MRI could identify esophageal cancer stages and lymph node metastasis before treatment initiation. Based on significant differences in the Rad-score, the radiomics signature successfully differentiated between ESCC stages I - II and III - IV before the preoperative period. In addition, by comparing the radiomics characteristics and those of the combined models, our results showed that MRI-based radiomics could predict lymph node metastasis of esophageal cancer before the preoperative period. These observations were based on MRI’s excellent soft tissue resolution and multimodal imaging, which can provide a high-quality image basis for accurate tumor delineation and radiomic analysis.
Surgery is the most essential treatment for esophageal cancer. However, operating on advanced esophageal cancer (~ stages) is challenging, and the recurrence rate is high after surgical treatment. The lymph node status in esophageal cancer is a significant independent predictor of prognosis, and the method used for lymph node dissection is crucial for esophageal cancer treatment [4]. Some studies have suggested that systematic lymph node dissection in esophageal cancer patients should be conducted only when the tumor invades the submucosa and that extended lymph node resection may increase postoperative complications [14-15]. In addition, lymph node staging is also crucial for implementing neoadjuvant therapy [16]. Therefore, it is essential to accurately predict the stage and lymph node metastasis in esophageal cancer patients before surgery to allow the formulation of a treatment plan and prognostic evaluation. CT is a common imaging method for preoperative esophageal cancer staging, but its effect on staging is poor. By extracting high-throughput features from medical images, radiomics can reveal tumor heterogeneity and biological behavior and improve the accuracy of tumor diagnosis and patient prognosis.
This study demonstrated the feasibility of predicting esophageal cancer's stage and lymph node metastasis with MR radiomic characteristics. We used T2WI and T1WI-Gd to extract 1316 quantitative image features from tumors. Eight features that contributed most to the differentiation of EC stages and nine that contributed most to the differentiation of lymph node metastasis were selected to construct the models. T2WI radiomics features are based on the extraction of water molecule signal strength, which primarily reflects the characteristics of the water content of the tumor.
The obtained high-order radiomics feature is mainly the gray level co-occurrence matrix, which reflects the gray level difference, including the gray level distribution uniformity and texture thickness. The more uneven the gray level and the more complex the texture, the greater the heterogeneity of the tumor. The histological features of the T1WI-Gd images were obtained based on the solid part of tumor enhancement and most reflected the tumor’s microvessel density, histological grading, and invasiveness [17]. The extracted higher-order features are more diverse, including the degree of difference between adjacent pixels, a gray area matrix, and dependence on the advantages of high gray, which can better reflect the heterogeneity of tumors in multiple dimensions [18].
As in other studies [19-21], we combined the radiomics characteristics of two sequence images to distinguish the esophageal cancer stages I - II and III - IV and lymph node metastasis and achieved satisfactory results (AUC = 0.884, 0.858 in the main cohort, AUC = 0.765, 0.711 in the verification cohort). Compared with previous studies on esophageal cancer tumor staging and lymph node metastasis, the prediction efficiency of the radiologic features as we built was better than that of the CT-based radiomics methods used for predicting esophageal cancer stages I - II and III - IV (AUC = 0.795 in the main cohort, AUC = 0.694 in the validation cohort) [22]. These could be because the model built by combining the radiomics features of the MR sequence images can mutually complement the image information and be more comprehensive, objectively reflecting tumor heterogeneity. In addition, our results were similar to those of other studies on the prediction of lymph node me tastasis of esophageal cancer based on MR radiomics characteristics (AUC = 0.821 in the main cohort, AUC = 0.762 in the validation cohort) [19], further confirming the feasibility of MR imaging characteristics to predict lymph node metastasis of esophageal cancer.
This study had several limitations. First, the sample size was relatively small, and our results should be further verified with a multicenter, large-sample study. Second, we only discussed whether the MRI radiomics could predict lymph node metastasis of esophageal cancer. However, the prediction efficiency of lymph node metastasis and its extent in different regions has not been studied. Again, a prospective, large-sample, multicenter prospective study is needed to verify our results. Last, the images in this study were all produced using the same MRI scanner. In a future study, we plan to discuss the influence of different types of equipment, field strength, and scanning parameters on the radiomics model.
In conclusion, this study explored the feasibility of using MR image-based radiomics methods to identify esophageal cancer stages I - II and III - IV and lymph node metastasis before treatment. We constructed a multi-feature radiomics model from the extracted radiomics features. This model has shown satisfactory performance in identifying esophageal cancer stages I - II and III - IV and lymph node metastasis early in surgery. With the popularization of magnetic resonance examination, we believe that MR-based radiomics might be a noninvasive and quantitative method for predicting the preoperative staging of esophageal cancer and guiding individual treatment decisions.
Materials and Methods
Patients
The clinical and MRI data of EC patients underwent lymph node dissection confirmed by surgery and pathology from January to December 2020 were retrospectively collected. The inclusion criteria were: The postoperative pathology was confirmed to be EC; Radical resection of esophageal cancer and lymph node dissection, with clear postoperative pathological stage and clear pathological lymph node results; MRI scan (T2WI and T1WI-Gd) was performed within two weeks before surgery; No EC treatment before MRI scan; and No previous history of other malignant tumors. The exclusion criteria were: Preoperative EC targeted for treatment; EC associated with other malignant tumors, and Poor MRI image quality, affecting observation and subsequent feature extraction. This study was approved by the Ethics Committee on Clinical Research and Novel Technologies of Meizhou People’s Hospital, and patient informed consent was waived for this retrospective study. All experiments were performed in accordance with relevant guidelines and regulations.
Ultimately, 120 patients with EC (89 males and 31 females, ages 44 to 82 years old, mean age 63.4±8.17 years) were included. Based on TNM staging, 58 patients were EC stages I - II (39 males, 19 females, mean age 63.4±8.4 years old), and 62 were EC stages III - IV (50 males and 12 females, mean age 63.5±7.8 years old). According to the postoperative lymph node pathology results, 56 patients were negative (38 males, 18 females,mean age 63.1±8.6 years old), and 64 patients were positive (51 males and 13 females, mean age 63.8±7.8 years old). The clinicopathologic characteristics of the patients are presented in Table 1.
Characteristics
P Value
LN status [n (%)]
P Value
I-II stage
III-IV stage
LN (+)
LN (-)
Age (¯x ± s) (year)
63.4±8.4
63.5±7.8
0.949
63.8±7.8
63.1±8.6
0.64
Gender
0.094
0.14
Male
39 (67.2)
50 (80.6)
51 (79.7)
38 (67.9)
Female
19 (32.8)
12 (19.4)
13 (20.3)
18 (32.1)
Location
0.597
0.64
Upper
4 (6.9)
3 (4.8)
3 (4.7)
4 (7.2)
Middle
33 (56.9)
31 (50.0)
32 (50.0)
32 (57.1)
Lower
20 (34.5)
22 (35.5)
23 (35.9)
19 (33.9)
Middle and lower
1 (1.7)
6 (9.7)
6 (9.4)
1 (1.8)
Pathological type
0.573
0.39
Squamous cell carcinoma
55 (94.8)
61 (98.4)
63 (98.4)
53(94.6)
Adenocarcinoma
1 (1.7)
0 (0)
0 (0)
1 (1.8)
Mixed carcinoma
2 (3.5)
1 (1.6)
1 (1.6)
2 (3.6)
Table 1: Characteristics of patients for TNM stages and LN status.
Image Acquisition
Imaging was performed with a 3.0T Siemens (Erlangen, Germany) Skyra MR scanner equipped with 18-channel phased array coil. The patient was placed supine and told to breathe as calmly as possible to reduce motion artifacts. The scanning range was from the level of the supraclavicular fossa to the gastric cardia. The scanning sequence and parameters were: (1) T2WI: slice thickness 3 mm, TR 3000 ms, TE 91 ms, NEX=1, matrix 256×256, field of view (FOV) 210 mm×210 mm and scanning time 2 min; and (2) T1WI-Gd: slice thickness 1.5 mm, TR 4.4 ms, TE 2.0 ms, NEX=1, matrix 160×160, FOV 150 mm×150 mm, and flip angle 12. During enhanced scanning, Gd-DTPA contrast agent was injected intravenously through the elbow via a high-pressure syringe at a 0.1 mmol/kg dose. The scanning time was 3 minutes 26 seconds.
Tumor Segmentation
Manual segmentation of the EC was performed on each patient's MR images utilizing an A.K. (Artificial Intelligent Kit), a new application platform for the overall solution of artificial intelligence (AI) in medical imaging radiomics launched by GE Healthcare. Two experienced associate senior radiologists (readers 1 and 2) carefully contoured the tumor on all T2WI and TIWI-Gd images to generate two 3D segmentations of the entire tumor. These regions of interest (ROIs) were performed for subsequent feature extraction and further analysis.
Radiomic Feature Extraction
Patients were randomly divided into primary (84 patients) and validation (36 patients) cohorts. Radiomic features were calculated based on the segmentation results from the primary cohort using a homemade program in the A.K. For each MRI sequence, the 1316 extracted features covered the major feature pool in recent radiomic studies. The features were categorized into six primary types: (1) histogram features; (2) form factor features; (3) Gray Level Co-occurrence Matrix (GLCM) and Harilick features; (4) Gray Level Run Length Matrix (GLRLM) features; (5) Grey Level Size Zone Matrix (GLSZM) features; and (6) gray level dependence matrix (GLDM) features (Figure 1).
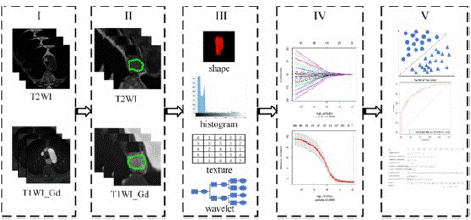
Figure 1: Flowchart of this study: (I) Original MR images: T2WI and T1WI_Gd. (II) Segmentation was performed on both T2WI and T1WI_Gd images to define the tumor region. (III) Radiomic features were extracted from the tumor region, including shape, first-order histogram, texture and wavelet group analysis. (IV) Several features were selected to build the radiomic signature using the minimal redundancy maximal relevance (mRMR) and the Least Absolute Shrinkage and Selection Operator (LASSO) method. (V) Finally, the classification ability of the radiomic signature was tested by the Receiver-Operating Characteristics (ROC) curves in both the primary and validation cohort.
The appropriate feature selection procedure was used to simplify the building model and avoid over-fitting issues. In addition, the minimal redundancy maximal relevance (mRMR) and Least Absolute Shrinkage and Selection Operator (LASSO) method were used for feature dimensionality reduction. For tuning coefficients λ and a, the respective minimum standard deviation and maximum AUC criteria were followed. For accurate prediction of the EC stages, eight features selected from the T2WI with T1WI-Gd images made the greatest contribution. On the other hand, nine features selected from T2WI with T1WI-Gd images were most useful for precise detection of LN metastasis. Then, each patient’s two radiomic signature sets were inputted into the logistic regression model. After the training in the primary cohort, a linear combination of the selected features was extracted. The sums of those linear combinations formed the radiomic signatures of each patient.
Statistics Analysis
The statistical analysis was performed in R (version 3.5.1; http://www.Rproject.org). The Shapiro-Wilk test was used to test whether the quantitative data were normally distributed. The Χ2-test was used to compare the differences in age, sex, tumor location, and pathological type between patients with EC stages I - II and stages III – IV and positive and negative LN metastasis. In addition, the primary cohort was used to train the logistic regression model, and the validation cohort was used to test the model. Finally, the Receiver operating Characteristic Curve (ROC), Sensitivity (SEN), and Specificity (SPE) were used to evaluate the performance of the prediction model, and the DeLong test was used to measure the significance of the Area Under the Curve (AUC) between the single-sequence and mixed-sequence models. P < 0.05 indicated statistical significance.
Author Statements
Acknowledgments
We would like to thank Gang Xiao (Hanshan Normal University, Chaozhou, China) for his assistance with statistical analysis. Indebtedness also goes to Dai-qiu Chen, with the English Dept. of College of Foreign Studies, Southern Medical College, for his English polishing.
Author Contributions
All authors participated in the study design, interpretation of the data, and critically reviewing the paper.Guihua Jiang put forward the study concepts and Rihui Yang wrote the first draft, and contributed to the writing of subsequent versions. Data was acquired and prepared for analysis by Ting Dong and Tianhui Zhang. Statistical analyses and preparation of tables and figures was performed by Weixiong Fan and Haodong Qin.Haiyang Dai was responsible for project administration and supervision.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflict of Interest statement
The authors have no relevant conflicts of interest to disclose.
Funding
This work was supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2020A1515110563), Medical Research Foundation of Guangdong Province (B2021052).
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA: A Cancer Journal for Clinicians. 2022; 72: 7-33.
- Wild CP, Weiderpass E, Stewart BW. World cancer report: cancer research for cancer prevention. Lyon: International Agency for Research on Cancer. 2022: 23-33.
- Liu S, Zheng H, Pan X, Chen L, Shi M, Guan Y, et al. Texture analysis of CT imaging for assessment of esophageal squamous cancer aggressiveness. J Thorac Dis. 2017; 9: 4724-4732.
- Rice T, Ishwaran H, Hofstetter W, Schipper PH, Kesler KA, Law S, et al. Esophageal Cancer: Associations With (pN+) Lymph Node Metastases. Annals of Surgery. 2017; 265: 122-129.
- Coroller TP, Agrawal V, Huynh E, Narayan V, Lee SW, Mak RH, et al. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. J Thorac Oncol. 2017; 12: 467–476.
- Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic revie. World J Gastroenterol. 2018; 14: 1479-1490.
- Goense L, Heethuis SE, Rossum P, Voncken FEM, Lagendijk JJW, Lam MGEH, et al. Correlation between functional imaging markers derived from diffusion-weighted MRI and 18F-FDG PET/CT in esophageal cancer. Nuclear Medicine Communications. 2017; 39: 60-67.
- Liu J, Wang Z, Shao H, Qu D, Liu J, Yao L, et al. Improving CT detection sensitivity for nodal metastases in oesophageal cancer with combination of smaller size and lymph node axial ratio. European Radiology. 2018; 28: 188-95.
- Liu J, Wang Z, Shao H, Qu D, Yao L. Improving CT detection sensitivity for nodal metastases in oesophageal cancer with combination of smaller size and lymph node axial ratio. European Radiology. 2017; 28: 188-195.
- Radiation Tumor Therapy Doctor Branch of Chinese Medical Doctor Association, Radiation Oncology Branch of Chinese Medical Association, Cancer Radiotherapy Committee of China Anti-Cancer Association. Chinese Guidelines for Radiotherapy of Esophageal Cancer (2021 Edition). International Journal of Oncology. 2022; 49: 12-25.
- Wu S, Zheng J, Li Y, Yu H, Shi S, Xie W, et al. A Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Bladder Cancer. Clinical Cancer Research an Official Journal of the American Association for Cancer Research. 2017; 23: 6904-6911.
- Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. Science Foundation in China. 2016; 34: 2157-2164.
- Shi J, Dong Y, Jiang W, Qin F, Wang X, Cui L, et al. MRI-based peritumoral radiomics analysis for preoperative prediction of lymph node metastasis in early-stage cervical cancer: A multi-center study. Magnetic Resonance Imaging. 2022; 88: 1-8.
- Visser E, van Rossum PSN, Ruurda JP, van Hillegersberg R. Impact of lymph node yield on overall survival in patients treated with neoadjuvant chemoradiotherapy followed by esophagectomy for cancer: a population-based cohort study in the Netherlands. Ann Surg, 2017; 266: 863-869.
- Booka E, Takeuchi H, Nishi T, Matsuda S, Kaburagi T, Fukuda K, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine (Baltimore). 2015; 94: e1369.
- Van RP, Van HR, Lever FM, Lips IM, van Lier ALHMW, Meijer GJ, et al. Imaging strategies in the management of oesophageal cancer:what’s the role of MRI. Eur Radiol. 2013; 23: 1753-1765.
- Gao Y, Xu YS, Feng W, et al. Research advancements of dynamic contrast enhanced MRI quantitative parameters and histogram in breast cancer. Chinese Journal of Interventional Imaging and Therapy. 2021; 18: 183-186.
- Brown AM, Nagala S, Mclean MA, Lu Y, Scoffings D, Apte A, et al. Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI. Magn Reson Med. 2016; 75: 1708-1716.
- Qu J, Shen C, Qin J, et al. The MR radiomic signature can predict preoperative lymph node metastasis in patients with esophageal cancer. Eur Radiol. 2019; 42: 253-254.
- Hou Z, Li S, Ren W, Liu J, Yan J, Wan S. Radiomic analysis in T2W and SPAIR T2W MRI: Predict treatment response to chemoradiotherapy in esophageal squamous cell carcinoma. J Thorac Dis. 2018; 10: 2256-2267.
- Peter SN, Van Rossum, Cai Xu, Fried DV, Goense L, Court LE, et al. The emerging field of radiomics in esophageal cancer: current evidence and future potential.Transl Cancer Res. 2016; 5: 410-423.
- Wu L, Wang C, Tan X, Cheng Z, Zhao K, Yan L, et al. Radiomics approach for preoperative identification of stages I-II and III-IV of esophageal cancer. Chin J Cancer Res. 2018; 30: 396-405.