
Case Report
Austin J Radiol. 2024; 11(4): 1242.
Treatment of Anal Canal Carcinoma with Lung Metastasis by Implantation of 125I Seeds: Case Report
Wang C¹; Zhou C¹; Chen ZQ²; Li HB¹; Wang J³*
¹Department of Nuclear Medicine, Taizhou Hospital, China
²Department of Radiology, Wulian People’s Hospital, China
³Department of Nuclear Medicine and Medical PET Center, Second Hospital of Zhejiang University School of Medicine, China
*Corresponding author: Jing WANG, Department of Nuclear Medicine and Medical PET Center, Second Hospital of Zhejiang University School of Medicine, #88 Jiefang Road, Hangzhou, 310009, Zhejiang, China. Tel: 86-571-87767138; Fax: 86-571-87767188 Email: wangjing5678@zju.edu.cn
Received: August 22, 2024 Accepted: September 06, 2024 Published: September 16, 2024
Abstract
The use of radioactive 125I seed implantation in the treatment of lung metastases has shown a good prospect for treatment of metastatic lesions, especially in patients who cannot tolerate surgery or side effects of chemotherapy and/or radiotherapy. In this case of non-responsive anal canal carcinoma with lung metastasis after chemo-radiotherapy, the implantation of radioactive 125I particles was proved effective, not only in decreasing the lesion, but also in bringing less side effect to the patient. Expect for irradiation in short distance and long half-life of 125I particle, anti-tumor immune responses induced by radioactive 125I seed might be one of the reasons of good clinical response.
Keywords: Brachytherapy; 125I seed; Lung cancer metastases
Abbreviations: CRT: Chemoradiotherapy; PR: Partial Remission; ESMO: European Society for Medical Oncology; ESSO: European Society of Surgical Oncology; ESTRO: European Society for therapeutic radiology and Oncology; EDTMP: Ethylenediamine-Tetramethylene Phosphonic Acid; PSA/TRICOM: PROSTVAC; CRPC: Castrate-Resistant Prostate Cancer
Introduction
Anal canal carcinoma is mostly a local-regional cancer, with a metastatic potential in only 15% of patients [1]. Considering its high metastatic capacity after operative resection, in most of the cases, radiotherapy and chemotherapy are the preferable treatment methods. However, chemotherapy has a large side effect on the whole body, and the traditional external radiation therapy is also limited because the adjacent normal lung tissue is fragile under radiation. The use of radioactive 125I seeds implantation in the treatment of lung metastases has been reported, shown a good prospect for treatment of metastatic lesions [2]. For patients who cannot undergo surgical resection, radiotherapy and chemotherapy, or for whom are not sensitive to those treatment methods, 125I seeds implantation can be used for the treatment of pulmonary metastases. Taizhou hospital hold by Taizhou Enze Medical Center (Industry group) in Zhejiang Province reports the following case of radioactive 125I seeds using in non-responsive anal canal carcinoma with lung metastasis after chemo-radiotherapy.
Case Presentation
In this article we present the case of a 95-year-old male patient, who was admitted in our hospital in March 2011 with "blood in the stool and anal lump”, diagnosis of anal canal cancer and laparoscopic Miles operation (abdominal perineal rectal cancer radical mastectomy) on March 28, 2011, intraoperative pathology showed "anal ulcer, adenocarcinoma of high differentiation, mucinous carcinoma (T4N0M0)". six months later, post-operative CT showed “pulmonary metastases in two pulmonary lower lobes’, followed by oral chemotherapy of Tegafur, Gimeracil and Oteracil Porassium Capsules. In April 2013, oral chemotherapy regimen changed into Xelodadue to the severe gastrointestinal side effect. According to the RECIST 1.0 standard, chest CT re-examination during chemotherapy revealed a progressive trend (PD). Pulmonary metastases puncture biopsies on 2013-06-06 and 2013-06-11, its pathology and immunohistochemistry staining confirmed the diagnosis: ‘metastases of adenocarcinoma" (Figure 1). The patient received chemotherapy of carmofur from June 19, 2013; palliative radiotherapy (GTV 60Gy/30F) for the mass in right lower lobes was delivered from June 24, 2013, followed by X-knife radiotherapy (DT 44Gy/10F) for the metastasis foci in the left lung. According to the RECIST 1.0 criteria, chest CT re-examination in August 2013 showed SD. In January 2014, the patient appeared cough, expectoration, accompanied by sputum with blood, weakness, fever and chills. CT scan showed “multiple nodules in two lungs grew in sizes compared to previous study; pneumonia; multiple mediastinal lymph nodes”. Palliative radiotherapy (GTV 50Gy/10f) for lung metastases restarted from Feb 24, 2014, accompanied with chemotherapy of capecitabine (1.5qm, 1.0qn d1-d14). Due to severe systemic toxicity, capecitabine was stopped on March 2, 2014.
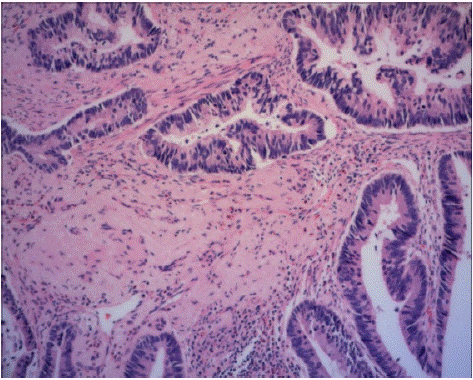
Figure 1: Pathological image of the patient (HE stained, paraffin sections).
Pathological results: anal ulcer of high differentiated adenocarcinoma, mucinous adenocarcinoma.
Considering the age of patient, as well as poor nutritional status and little response to the chemo-radiotherapy, implantation of radioactive 125I under the guidance of CT was performed on April 19, 2014. Prescription dose of 125I particles was 110 Gy; 125I particle activity is 0.7mCi, gamma ray energy is 27-35keV, tissue penetrating thickness is 1.7cm. 97 seeds of 125I particles were successfully implanted (Figure 2) in the largest metastases foci of the left lower lobe. According to the RECIST 1.0 criteria, chest CT re-examination in 3 and 8 months after the 125I implantation both revealed PR (partial remission) (Figure 3). The patient didn’t complain of severe discomfort untill the recent follow-up on March 31, 2015.
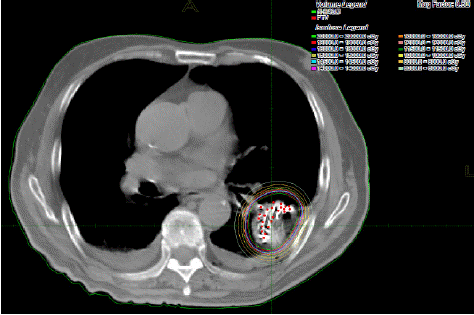
Figure 2: Implantation of 125I particles.
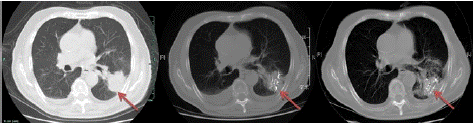
Figure 3: CT scans before and after implantation of 125I particles. Left: CT scan on April 11, 2014 (before implantation of 125I particles); Middle: CT scan on July 14, 2014 (3 months after implantation of 125I particles), PR (RECIST 1.0 criteria) compared to the previous study; Right: CT scan on December 22, 2014 (8 months after implantation of 125I particles), PR (RECIST 1.0 criteria) compared to the previous study.
Discussion
On July 6, 2014, ESMO (European Society for Medical Oncology) combined with ESSO (European Society of Surgical Oncology) and ESTRO (European Society for therapeutic radiology and Oncology) published in the official journal of the annals of Oncology (ANN Oncol) online: the "anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Anal cancer is a comparatively rare malignant tumor, in 2007 the United States has 4,650 cases of anal canal cancer patients, accounting for the 1.7% in tumors of digestive system. Anal canal adenocarcinoma is the origin of anal gland malignant tumors, the incidence was higher in male than that in female, the local recurrence rate and metastasis rate to be higher than that of squamous cell carcinoma of the anal canal. The biological behavior of anal canal adenocarcinoma is closer to the colorectal cancer. In most of the cases, abdominoperineal resection combined with post-surgical radiotherapy and 5-FU based chemotherapy is the preferable treatment regimen, 5-year survival rate of which is about 35%. While in the past 30 years, the treatment of anal canal cancer was re-evaluated. The CRT method (combined with radiotherapy and chemotherapy) had no doubt become the first choice in the treatment of anal canal cancer. The CRT regimen which was widely accepted was continuous radiotherapy (45Gy) combined with 2 cycles of 5-FU (W1, W5) and MMC (D1, D29) at the same time. For patients in T3-4, the additional radiation dose of 5.4Gy to 9.0Gy was recommended. After CRT, the distant metastasis incidence of anal carcinoma was between 10% ~ 17%. Lung is the most common metastasis organ, and the 5 years survival rate after distant metastasis is about 18%.
Brachytherapy is to implant radiation source into the tumor tissue to expose tumor cells to a continuous dose of radiation. Recently, using radioactive 125I particles for brachytherapy have been widely used in the treatment of a series of tumors, and showed very good effect [3]. It is reported that the efficiency of 125I particle implantation is much higher than external irradiation [4]. Radioactive 125I seed implantation is superior to the other treatment methods, mainly for the following reasons: 125I radioactive particle energy is not so high, but the gamma ray can be well concentrated in the target area. The small target volume and local high dose irradiation were closely related to the good therapeutic efficacy of 125I particles. During internal radiation, decreased the tumor size, comparatively increased the local dosage of 125I particles, therefore, the treatment effect was more significant [5]. In addition, the half-life of 125I is very long (59.4 days), so effective internal irradiation could last for a long time. Compared to the other radiotherapy, the effect of 125I particles on the surrounding normal tissues was much less. Because the radiation of 125I decreases with distance, and its gamma ray energy is exponentially decayed outside the target area. At a distance of 1cm and 2cm from the target, the acceptable dose decayed rapidly to about 20% and 5% [6], respectively.
Furthermore, except for local anti-tumor effect, local irradiation such as external beam radiation, radiolabeled monoclonal antibodies and brachytherapy can also reduce tumor growth outside the treatment field, often referred to as the abscopal effect [7]. Several studies [8,9] reported that abscopal effects were mediated by the radiation-induced immune modulation or induction of anti-tumor immune responses at higher doses of X-irradiation than 2Gy. Though the mechanisms and therapeutic potential of the abscopal effect have not been fully elucidated, there have been two main hypotheses concerning abscopal tumor regression: (a) Local irradiation induced a release of systemic cytokines that mediate a systemic antitumor effect and/or (b) Local irradiation induces systemic tumor specific T-cell responses. Treatment of tumors with 125I brachytherapy in a mouse model [10] resulted in a significant up-regulation of Fas, a death receptor in the signaling cascade required for cell death. Fas up-regulation has been previously demonstrated in a mouse model to be responsible for increased sensitivity to T-cell killing after irradiation. As our understanding of the immune modulatory effects of radiation has improved, interest in combining this type of therapy with immune-based therapies for the treatment of cancer has grown. A study that published in Lancet Oncology recently [11] reveals that combination of radiotherapy with granulocyte-macrophage colony-stimulating factor produced objective abscopal responses in 22% of non-small cell lung cancer patients and 36% of breast cancer patients. The combination of radiotherapy and immunotherapy is a promising therapeutic approach in patients with metastatic solid tumors. Clinically, an additional radioactive Samarium-153 was studied in a randomized phase II trial to check if 153Sm-EDTMP combined with an rV/rF-PSA-TRICOM vaccine can improve time to progression over 153Sm- EDTMP alone in patients with Castrate-Resistant Prostate Cancer (CRPC) metastatic to bone [12]. In a mouse model bearing both a subcutaneous tumor and pulmonary metastases, treatment of the primary tumor via 125I-brachytherapy seed implant combined with systematic vaccination mediated regression of the distant pulmonary metastases [10]. More clinical trials that evaluate the immune response induced by 125I seed brachytherapy and its combination with immunotherapy are warranted.
To conclude, in this case of non-responsive anal canal carcinoma with lung metastasis after chemo-radiotherapy, the implantation of radioactive 125I particles was proved effective, not only in decreasing the metastasis foci, but also in bringing less side effect to the patients. Recently, abscopal response that describes radiotherapy-induced immune-mediated tumor regression at sites distant to the irradiated field has been proved. The anti-tumor immune responses induced by radioactive 125I seed and the combination of brachytherapy with immunotherapy necessitate more clinical trials.
References
- Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983; 51: 1826-1829.
- Tanderup K, Ménard C, Polgar C, Lindegaard JC, Kirisits C, Pötter R. Advancements in brachytherapy. Adv Drug Deliv Rev. 2017; 109: 15-25.
- Li CX, Zhang FJ, Zhang WD, Zhang L, Huang ZL, Wu PH. Feasibility of 125I brachytherapy combined with sorafenib treatment in patients with multiple lung metastases after liver transplantation for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010; 136: 1633–1640.
- Li W, Zheng YF, Li YM, Guan J, Jiang JQ, Yu YK, et al. Oncol Lett. 2017; 14: 6690-6700.
- Mazeron JJ, Noël G, Simon JM. Head and neck brachytherapy. Semin Radiat Oncol. 2002; 12: 95-108.
- Yu Y, Anderson LL, Li Z, Mellenberg DE, Nath R, Schell MC, et al. Permanent prostate seed implant brachytherapy: report of the American Association of Physicists in Medicine Task Group No.64. Med Phys. 1999; 26: 2054-2076.
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009; 10: 718-26.
- Rödel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015; 356: 105-13.
- Camphausen K, Moses MA, Me´nard C, Sproull M, Beecken WD, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003; 63: 1990-3.
- Hodge JW, Sharp HJ, Gameiro SR. Abscopal Regression of Antigen Disparate Tumorsby Antigen Cascade After Systemic Tumor Vaccinationin Combination with Local Tumor Radiation. Cancer Biother Radiopharm. 2012; 27: 12-22.
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Kerimian MF, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015; 16: 795-803.
- 153Sm-EDTMP With or Without a PSA/TRICOM Vaccine to Treat Men with Androgen-Insensitive Prostate Cancer. [ hyperlinked with www.clinicaltrials.gov].