
Special Article: Radiologic Procedures
Austin J Radiol. 2024; 11(4): 1244.
Imaging of Ovarian Metastases (OM) from Breast Cancer: A Review
Diana Donatello*
Radiologist Independent Researcher, Costa Contina Street 19 Zip Code 66054, Vasto, Italy
*Corresponding author: Diana Donatello Radiologist Independent Researcher, Costa Contina Street 19 Zip Code 66054, Vasto, Italy. Email: dianadonatello@hotmail.it
Received: September 12, 2024 Accepted: September 18, 2024 Published: September 19, 2024
Abstract
The ovary is a common site of metastases from other primary malignancies, and 5-30% of ovarian cancers are metastatic malignancies [1-16]. The most common primary origins are the breast, colon, and stomach[24]. Although imaging cannot always differentiate between secondary and primary ovarian neoplasms, and pathologic confirmation is generally required, it is important to recognize suggestive imaging features on pelvic US, CT and MR imaging [5,8-10,16,18-20]. OM from breast cancers is frequently asymptomatic until the masses have grown to certain size, and the metastatic tumors are frequently manifested as bilateral, solid, hypervascular, small ovarian masses [2]. Even though the Immunohistochemistry plays a key role in distinguishing between primary ovarian tumors and OM, and it was also important for confirming the metastatic nature of the ovarian lesion and diagnosing the primary tumor, imaging is vital to guide radiologists to include metastases in their differential diagnosis for atypical adnexal masses and in some cases avoid unnecessarie mutilative surgery [2-24]. In this review i will analize the main radiology features of OM from breast cancer to direct the surgery planning including referral practice, selection of candidates for primary chemotherapy by demonstration of non resectable disease, and tissue sampling in case of peritoneal carcinomatosis.
Keywords: Ovarian Metastases; Breast Cancer; Ovarian Cancer
Aim
To try to differentiate by literature review the main radiological features (US, CT and MR imaging) of metastatic ovarian masses derivate from breast cancer from primary ovarian cancer and other meastatic ovarian disease.
Methods
Literature review
Discussion
Asymptomatic ovarian masses may be the first sign of OM, and indeed manypatients were diagnosed by the presentation of masses, which are commonly bilateral, solid, hypervascular and small [1-7,16]. When clinical signs indicate an ovarian tumor, transvaginal ultrasonography is the prime option [2,4,5,12,16,20-22,24]. The sonographic feature ‘lead vessel’, defined by Testa et al. as a primary vessel of tree-shaped morphology penetrate from the periphery of the ovarian mass into the center, can be considered as a characteristic of OM [6-16]. Patients with breast cancer are diagnosed at early stages, and patients occasionally have BRCA1/2 mutations (increasing their risk for both breast and primary ovarian cancers), accurate clinical history is particularly important for radiologists. In general, most adnexal masses among patients with a history of breast cancer are benign [16-19]. However, a new adnexal mass in a patient with stage 4 breast cancer is more likely to be a metastasis, while a similar finding in a patient with stage 1 disease is more likely due to a benign ovarian process [6]. Likewise, a BRCA- positive patient is more likely to present with primary ovarian malignancy than a metastasis. By imaging, OM from breast cancer are often bilateral, solid, small (=5cm), and hypervascular [11,16-19].
US
Figure 1 and 2 show representative ultrasound images of OM. From breast cancers with their typical solid pattern. The borders can be irregural according to Testa et al [5]. in an half of the cases that they observed. Absence of cystic fluid and or papillary projectionts, with an intermediate colour signal score. Presence of the “lead vessel” (the prevalence of a main peripheral vessel penetrating into the central part of the ovar
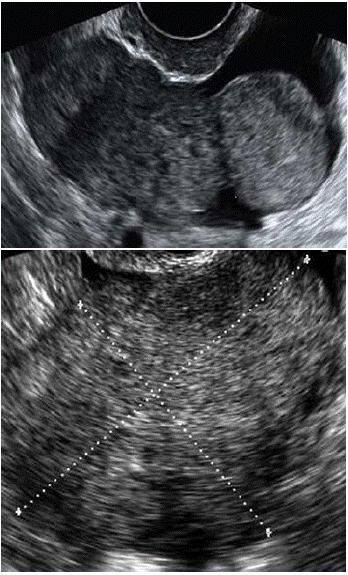
Figure 1 & 2: Transvaginal US: homogeneously solid lesions with sharp borders. The border is well visualized if the metastasis is surrounded by fluid in the pelvis. These metastases move freely when pressure is exerted on them by the vaginal probe. Sometimes hypoechoic irregular areas within the solid mass change their homogeneously solid echostructure; this feature probably reflects necrosis [5-10].
In Testa et al study [15] the presence of the lead vessel was detected in 11/31 (35.4%) metastatic tumors, and in only two (0.01%) cases of primary ovarian carcinoma in these two cases, histology indicated serous ovarian carcinoma and ovarian fibrosarcoma.
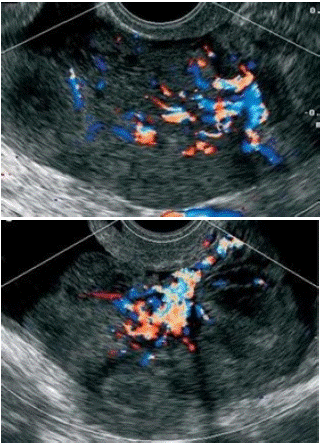
Figure 3 & 4: US appereance of the lead vessel in Testa et al study, the major vessel penetrete from the periphery into the central part of the ovarian mass with a tree-shaped morphology [15].
In our past sudies we observed how the lead vessel could be present in a case of primary ovarian lymphoma. (Figure 5 & 6) [23].
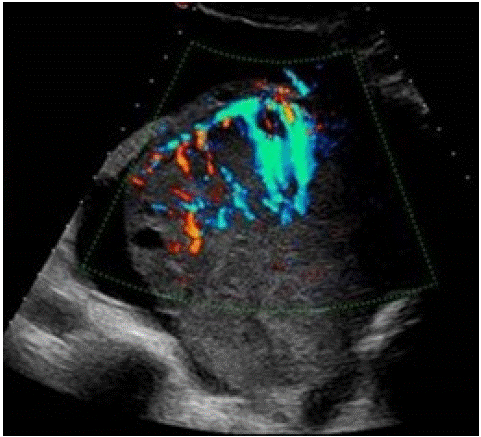
Figure 5: 44 yo female affected by NHL, Colour Doppler TV US shows a left ovarian mass with the “lead vessel” sign: a mainvessel with many thinner branching vessels entering from the periphery to the core of the mass; two an-hecoic follicles are visible at the periphery of the mass [23].
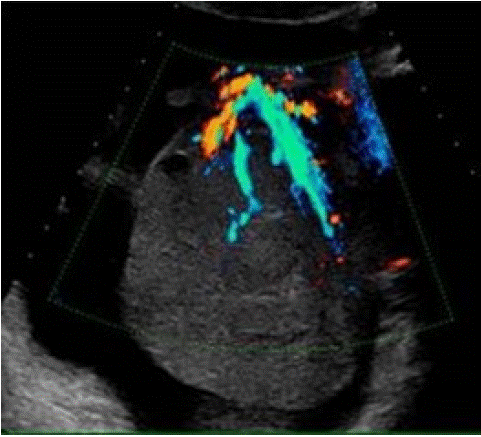
Figure 6: Same patient of Fig 5, TV Colour and Power Doppler TVS clearly depict the main vessel (the “lead vessel”) entering from the periphery to the centre of the ovarian mass, with many branching vessels of thinner width [23].
CT
On CT we can see soft tissue density with areas of cystic necrosis. On contrast, solid components demonstrate inhomogeneous enhancement.
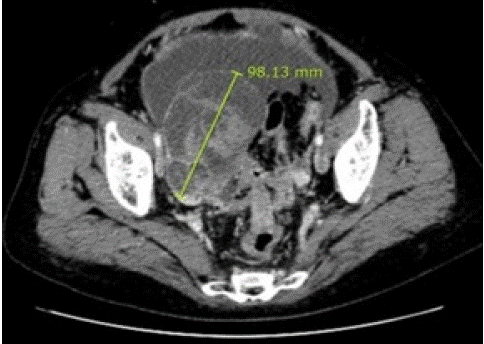
Figure 7: Abdominal-pelvic Computed Tomography (CT) imaging in a 61 y.o. woman with breast cancer revealed both a solid ovarian cystic tumor with numerable ascites in the abdominal cavity and disseminated peritoneal carcinomatosis [24].
MRI
On MRI solid components have heterogeneous T2 signal. If mass have a cystic component, thickened septations are uniformly present. Necrosis and heterogeneous enhancement are also common [18,19].
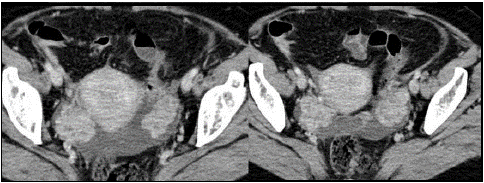
Figure 8: Heterogeneous solid masses, OM from breast cancer [25].
68Ga-FAPI PET/CT
FAPI, a newly developed tumor imaging target, can specifically bind to FAP in vivo, which is overexpressed in cancer-associated fibroblasts in many primary solid tumors and metastases OM of breast cancer can present with clinical and imaging characteristics resembling primary malignancy. We know that PET/CT can detect regional metastatic lymph nodes and distant metastasis through one-stop imaging, which is becoming more significant in clinical staging and follow-up of tumors. Many recent studies have demonstrated that 68Ga-FAPI is superior to 18F-FDG in the detection of many types of tumors [13]. For malignant tumors in the abdomen and the pelvis, such as gastrointestinal tumors and peritoneal carcinoma, whether primary or metastatic, 68Ga-FAPI may have a greater advantage over 18F-FDG, with a higher detection rate [13,14].
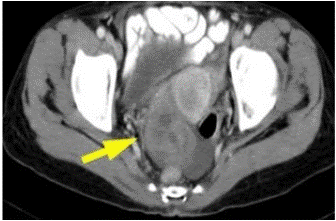
Figure 9: Contrast-enhanced axial pelvic CT showed complex cystic tumors involving the ovaries (arrow) [26].
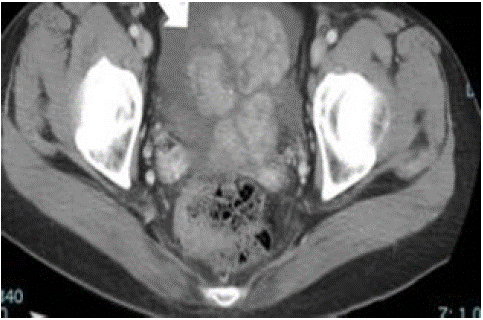
Figure 10: 54-year-old woman, breast invasive lobular carcinoma (T3N2aM1) with OM. CT scan of pelvis revealed an ovarian tumor with increased ascite [24].
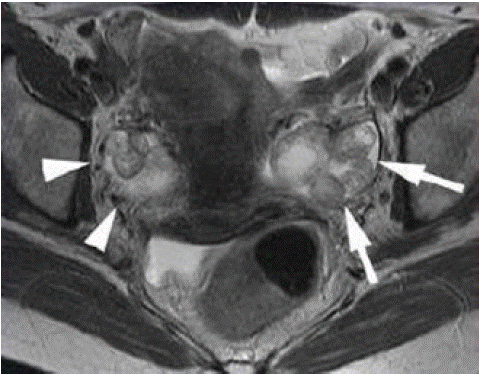
Figure 11: Fast spin-echo axial T2 MRI image shows a small, multi-nodular bilateral ovarian tumor (right arrowheads and left arrowheads). The tumor in the left ovary has a stronglyintense central area [19].
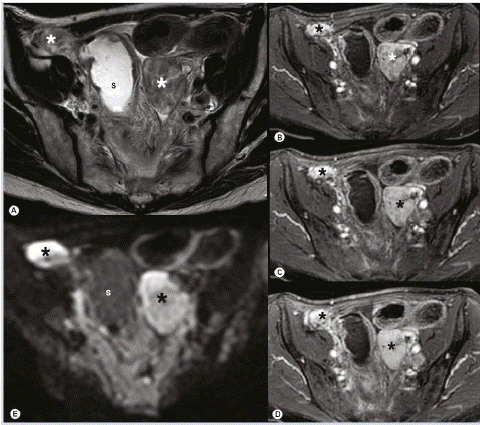
Figure 12: Bilateral OM in a patient with a history of breast cancer. (A) Bilateral solid adnexal masses (asterisk) with inhomogeneous intermediate to low signal intensity are visualized on transaxial T2-weighted imaging. (B–D) The early and avid contrast uptake acquired in a dynamic series within 2 min and (E) complementary high signal intensity on high b-value diffusionweighted MRI allow for diagnosis of metastases and are not found in benign stromal tumors. S: Sigmoid colon [27].
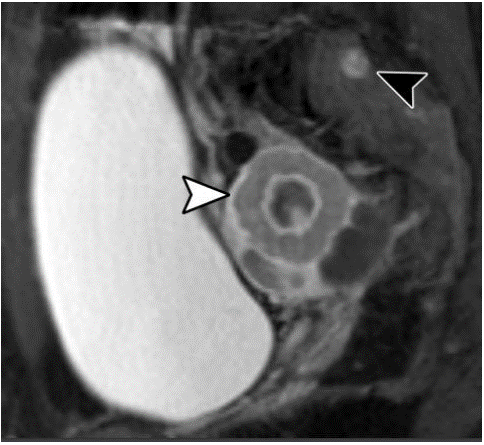
Figure 13: Sagittal postcontrast fat sat-T1WI in a 37 yo woman with a history of breast cancer shows a solid right ovarian mass (white arrowhead) with central necrosis, and a sacral bone metastasis (black arrowhead) [19].
Conclusion
• OM from breast cancer is represented in most of cases by:
• Homogeneusly solid masses
• Bilateral masses
• On US presence of hypoecoic areas in the masses that reflects necrosis
• On US presence of “lead vessel”
• On CT: heterogeneus complex mass with an area of central necrosis
• On MRI: heterogeneous T2 signal, inhomogeneous intermediate to low signal intensityT2-weighted imaging, early and avid contrast uptake in a dynamic series and high signal intensity on high b-value diffusion weighted MRI.
• Possible visualization of areas of central necrosis both in CT sequences and MRI sequences
Possible visualization of physiologic ovarian cysts or preserved ovarian follicles in the cortex
References
- Akizawa Y, Kanno T, Horibe Y, Shimizu Y, Noguchi E, Yamamoto T, et al. Ovarian metastasis from breast cancer mimicking a primary ovarian neoplasm: A case report. Mol Clin Oncol. 2021; 15: 135.
- Wang J, Tian W, Zhou Y, Zhang X, Deng Y. Ovarian metastasis from breast cancer in three Chinese females: Three case reports. Medicine (Baltimore). 2019; 98: e15395.
- Willmott F, Allouni KA, Rockall A. Radiological manifestations of metastasis to the ovary. J Clin Pathol. 2012; 65: 585-90.
- Hann LE, Lui DM, Shi W, Bach AM, Selland DL, Castiel M. Adnexal masses in women with breast cancer: US findings with clinical and histopathologic correlation. Radiology. 2000; 216: 242-7.
- Testa AC, Ferrandina G, Timmerman D, Savelli L, Ludovisi M, Van Holsbeke C, et al. Imaging in gynecological disease (1): ultrasound features of metastases in the ovaries differ depending on the origin of the primary tumor. Ultrasound Obstet Gynecol. 2007; 29: 505-11.
- Tian W, Zhou Y, Wu M, Yao Y, Deng Y. Ovarian metastasis from breast cancer: a comprehensive review. Clin Transl Oncol. 2019; 21: 819-827.
- Pimentel C, Becquet M, Lavoue V, Henno S, Leveque J, Ouldamer L. Ovarian Metastases from Breast Cancer: A Series of 28 Cases. Anticancer Res. 2016; 36: 4195-200.
- Forstner R. Radiological staging of ovarian cancer: imaging findings and contribution of CT and MRI. Eur Radiol. 2007; 17: 3223-35.
- Brown DL, Zou KH, Tempany CM, Frates MC, Silverman SG, McNeil BJ, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology. 2001; 219: 213-8.
- Karaosmanoglu AD, Onur MR, Salman MC, Usubutun A, Karcaaltincaba M, Ozmen MN, et al. Imaging in secondary tumors of the ovary. Abdom Radiol (NY). 2019; 44: 1493-1505.
- Wang J, Wang B. Metastasis of Ovarian Cancer to Breast: A Case Report and Review of Imaging Manifestations. Cancer Manag Res. 2020; 12: 13015- 13021.
- Cerkauskaite D, Zilinskas K, Varnelis P, Oreibi ME, Asejev V, Dulskas A. Ovarian metastases from breast cancer: A report of 24 cases. J Gynecol Obstet Hum Reprod. 2021; 50: 102075.
- Li T, Jiang X, Zhang Z, Chen X, Wang J, Zhao X, et al. Case Report: 68Ga- FAPI PET/CT, a more advantageous detection mean of gastric, peritoneal, and ovarian metastases from breast cancer. Front Oncol. 2022; 12: 1013066.
- Kitajima K, Suzuki K, Senda M, Kita M, Onishi Y, Maeda T, et al. FDG PET/CT features of ovarian metastasis. Clin Radiol. 2011; 66: 264-8.
- Testa AC, Mancari R, Di Legge A, Mascilini F, Salutari V, Scambia G, et al. The ‘lead vessel’: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008; 31: 218-21.
- Alcazar Garcia-Manero. Transvaginal gray scale and color doppler sonography in primary ovarian cancer and metastatic tumors to the ovary. J Ultrasound Med. № 22, ?. 243.
- Shimaa Abdalla Ahmed, Hanan Ahmed El Taieb. Variations in radiological features between primary and secondary ovarian malignancies. The Egyptian Journal of Radiology and Nuclear Medicine. 2018; 49: 828- 837.
- Roseland ME, Millet JD, Wasnik AP. Imaging of Metastatic Disease to the Ovary/Adnexa. Magn Reson Imaging Clin N Am. 2023; 31: 93-107.
- Koyama T, Mikami Y, Saga T, Tamai K, Togashi K. Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation. Abdom Imaging. 2007; 32: 784-95.
- McCluggage WG, Wilkinson N. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005; 47: 231-47.
- Xu Y, Yang J, Zhang Z, Zhang G. MRI for discriminating metastatic ovarian tumors from primary epithelial ovarian cancers. J Ovarian Res. 2015; 8: 61.
- Lee SJ, Bae JH, Lee AW, Tong SY, Park YG, Park JS. Clinical characteristics of metastatic tumors to the ovaries. J Korean Med Sci. 2009; 24: 114-9.
- Donatello D, Battista G, Sassi C. Imaging of ovarian lymphoma. J Ultrasound. 2023; 26: 799-807.
- Lee MI, Jung YJ, Kim DI, Paik HJ, Lee S, Jung CS, et al. Metastasis to breast from ovarian cancer and primary ovarian cancer concurrently diagnosis. Gland Surg. 2021; 10: 1806-1811.
- A Castan, A Mir Torres, G Riazuelo, I Escartin, C Ospina, C Rodríguez, et al. Ovarian pathology: A practical approach to imaging diagnosis and management. ECR 2015 / C-1865.
- Huang PC, Wu RC, Juan YH, Ho HY, Lin YC, Huang YT, et al. Diagnostic Accuracy of Whole-Body Computed Tomography for Incidental Ovarian Tumors in Patients with Prior Breast Cancer. Diagnostics (Basel). 2022; 12: 347.
- Rosemarie Forstner, Matthias W Meissnitzer, Alexander Schlattau, John A Spencer. MRI in ovarian cancer. Review Article - Imaging in Medicine. 2012: 4.