Special Article - Surgery
Austin J Radiol. 2020; 7(3): 1113.
Fetal Bronchopulmonary Sequestration: Case Series and Review of the Literature
Litwinska M¹*, Litwinska E², Janiak K¹, Piaseczna-Piotrowska A³ and Szaflik K¹
¹Department of Gynecology, Polish Mother’s Memorial Hospital - Research Institute, Poland
²Department of Perinatology and Gynecology,Polish Mother’s Memorial Hospital - Research Institute, Poland
³Department of Pediatric Surgery and Urology, Polish Mother’s Memorial Hospital - Research Institute, Poland
*Corresponding author: Magdalena Litwinska, Department of Gynecology, Fertility and Fetal Therapy, Polish Mother’s Memorial Hospital - Research Institute, ul. Rzgowska 281/289, 93-338 Lodz, Poland
Received: June 10, 2020; Accepted: July 07, 2020; Published: July 14, 2020
Abstract
Objective: To evaluate the prenatal course and perinatal outcome of fetuses with bronchopulmonary sequestration (BPS) managed expectantly or using minimally-invasive methods depending on the presence of fetal hydrops.
Materials and Methods: This was a retrospective study of 18 fetuses with suspected bronchopulmonary sequestration managed between 2006 and 2018 in a tertiary fetal therapy center. Medline was searched to identify cases of BPS managed expectantly or using minimally-invasive methods.
Results: In ten fetuses with BPS, at the time of initial diagnosis, there was no evidence of cardiac compromise. These fetuses were managed expectantly. Partial regression of the lung lesion, no change and progression of the tumor’s size were stated in 6(60%), 3(30%) and 1(10%) case respectively. All infants were born at term; seven required sequestrectomy. Eight hydropic fetuses with BPS were qualified to intrauterine intervention: thoraco-amniotic shunt was inserted in three fetuses, laser coagulation of the feeding vessel was performed in four cases and one fetus had combined treatment consisting of laser coagulation and thoraco-amniotic shunt. All infants were born at term and four required sequestrectomy. In previous series of various percutaneous interventions for BPS associated with hydrops the survival rate was 89% (26/29) for thoraco-amniotic shunting, 97.8% (45/46) for laser coagulation of the feeding vessel, 75% (3/4) for intratumor injection of sclerosant. The rate of preterm birth before 37 weeks in the group treated with laser was (6/42) 16.7% compared to (21/39) 63.6% in the group treated with thoraco-amniotic shunting. The need for postnatal sequestrectomy was lower in the group of fetuses treated with laser 8/25 (32%) in comparison to fetuses treated by thoraco-amniotic shunting 18/22 (81%).
Conclusion: In fetuses with BPS without hydrops, progression of the lesion’s volume leading to cardiac compromise is unlikely. Therefore, the prognosis is favorable and expectant management justified. In hydropic fetuses with BPS, intrauterine therapy using minimally invasive methods, prevents fetal demise. Both, the rate of preterm birth and the need for postnatal surgery is significantly lower in the group treated with laser compared to the group treated with thoraco-amniotic shunting.
Keywords: Bronchopulmonary sequestration; Fetal therapy; Thoracoamniotic shunt; Laser Coagulation of the feeding vessel
Introduction
Bronchopulmonary Sequestration (BPS) is a rare disorder of the lower respiratory tract, presenting as a solid lesion receiving blood supply from the systemic artery. The prognosis of this condition is generally favorable, unless there is associated hydrothorax or hydrops which is thought to be a consequence of impaired cardiac function due to mediastinal shift and compression of systemic veins [1].
In the case of large lesions with hydrops, several attempts at percutaneous fetal intervention have been described, with the aim of improving perinatal outcome. These include: thoracocentesis [1- 6], placement of thoraco-amniotic shunt [1,7-20], laser coagulation of the feeding vessel [18,21-29], intratumor injection of sclerosant [30,31] and combined treatment [29,32]. Although, the condition is rare and therefore the reported data are limited, it is evident that invasive management in hydropic fetuses is beneficial.
The objective of this study is firstly, to report our experience with the management of 18 fetuses diagnosed with BPS with and without associated hydrothorax and/or hydrops and secondly, to review the literature on this kind of pathology.
Materials and Methods
This was a retrospective study of 18 fetuses diagnosed with bronchopulmonary sequestration in our Fetal Therapy Center between 2006 and 2018. Within this period of time, 102 fetuses with various echogenic lung lesions were referred to our center for further diagnosis and management. In this group, 20 fetuses were suspected for BPS, but two fetuses were excluded from the study because of additional abnormalities. Out of 18 fetuses with BPS, eight met qualification criteria to fetal therapy of first, presence of a large solid lesion receiving blood supply from a clearly identifiable vessel originating from aorta, second, presence of hydrothorax and/ or hydrops and third, absence of other major defects.
Preoperatively, a detailed ultrasound examination was carried out to exclude any other major defects and to determine the presence and severity of pleural effusion and hydrops, as well as to measure the CVR. Essentially, the volume of the lesion was calculated for the maximum transverse, anterioposterior and longitudinal diameter and then divided by the head circumference [CVR = (length × width × height of the lesion × 0.52) / head circumference]. Specialist fetal echocardiography was also carried out to exclude cardiac defects, assess size of the heart and muscle contractility, diagnose possible atrio-ventricular regurgitation and evaluate the blood flows using Doppler techniques.
All patients received detailed counselling by a fetal medicine specialist and a pediatric surgeon concerning the nature of the lesion and likely prognosis. In case of fetuses which met qualification criteria to intrauterine therapy, written consent form for thoraco-amniotic shunting or laser coagulation of the feeding vessel was taken after counselling about possible benefits, risks and complications from this kind of treatment.
Up to January 2013, cases of BPS with hydrops/hydrothorax were treated with thoraco-amniotic shunts. Thereafter, they were treated with laser coagulation of the feeding vessel.
Thoraco-amniotic shunt insertion was conducted using the technique first described by Rodeck et al. [2]. Ultrasound scanning was used to obtain a transverse section of the fetal thorax and define the appropriate site of entry on the maternal abdomen which was infiltrated with local anesthetic (10mL of 10% lignocaine) down to the myometrium. Under continuous ultrasound guidance, a metal cannula with a trochar (external diameter 3mm, length 15cm; Rocket KCH Reusable Introducer Set Washington, United Kingdom) was introduced transabdominally into the amniotic cavity and inserted through the fetal chest wall into the pleural cavity. The trochar was then removed and the shunt (diameter 2mm, length 12cm; Rocket KCH Fetal Bladder Catheter, Washington, United Kingdom) was inserted into the cannula. A short introducer rod is then used to deposite half of the catheter into the pleural cavity. Subsequently, the cannula was gradually removed into the amniotic cavity where the other half of the catheter was pushed by a longer introducer.
Laser coagulation of the feeding vessel was conducted under ultrasound guidance. The cross-section of the fetal thorax was visualized using ultrasound scanning and a 18-G needle was introduced through the fetal thorax. A 0.7 mm laser fiber was than inserted through the needle with its tip pointing directly the feeding artery. The vessel was coagulated for 6-12 seconds using the output of 40-50 W. The procedure was repeated three times until the absence of blood flow using color Doppler was confirmed.
In cases of polyhydramnios (amniotic fluid index >25 cm) amniodrainage was carried out through the cannula/needle. Perioperative tocolysis was provided by betamimetic if the gestation was >24 weeks. All patients received antibiotic prophylaxis (Penicillin 1.2g IV).
In case of fetuses qualified to expectant management, serial ultrasound scans every 2 weeks were offered. Fetuses managed with minimally-invasive methods were followed every day for the first week and every 1-2 weeks thereafter until delivery to confirm the resolution of hydrops and lack of its recurrence. After delivery the chest drains were immediately clamped or removed to avoid development of pneumothorax.
The perinatal data of the fetuses which underwent the intrauterine procedures were obtained from the database of the Polish Mother’s Memorial Research Institute. The fetal and newborn characteristics included in the analysis were intrauterine death, gestational age and postnatal follow-up including surgery.
Literature Search
Searches of Medline and Embase were performed to identify all studies in the English language that reported on the expectant and invasive management of fetuses diagnosed with BPS. In case of fetuses managed expectantly only reports of at least two foetuses were considered [1,11,18-20,23,33-44].
Results
The characteristics of the 18 fetuses with BPS managed in our Fetal Therapy Center are summarized in Table 1 (expectant management) and Table 2 (minimally-invasive treatment).
Table 1: Data of 10 fetuses with BPS managed expectantly.
Case
Findings at the time of surgery
Type of Intervention
Follow up
GA
Side
Type
Vessel
CVR
AFI
Mediastinal shift /
hydrops
Shunt (no.)
Laser
Outcome
Birth
Apgar
Surgery
1
21
Right
Extralobar
TDA
1.49
28
yes
Shunt x 2
LB
36
9
7
2
23
Left
Extralobar
TDA
2.01
30
yes
Shunt x 1
LB
34
8
7
3
20
Left
Extralobar
TDA
1.62
26
yes
Shunt x 2
LB
38
9
10
4
20
Left
Extralobar
TDA
1.56
28
yes
Shunt x 1
Laser
LB
40
10
6
5
23
Left
Extralobar
TDA
1.79
24
yes
Laser
LB
39
9
2
6
21
Left
Extralobar
TDA
1.42
24
yes
Laser
LB
39
10
No intervention
7
24
Right
Extralobar
TDA
2.88
28
yes
Laser
LB
41
10
No intervention
8
25
Right
Extralobar
TDA
3.36
31
yes
Laser
LB
40
9
No intervention
GA: Gestational Age in weeks; TDA: Thoracic Descending Aorta; AFI: Amniotic Fluid Index; CVR: Congenital Pulmonary Airway Malformation Volume Ratio; LB: Livebirth; Birth: Gestational Age At Birth in weeks; Surgery: Age at Postnatal Surgery in months.
Table 2: Data of 4 fetuses with BPS managed with intrauterine intervention.
Fetuses managed expectantly
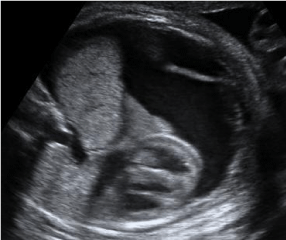
The median gestational age at the time of initial diagnosis was 21.5 (range 20-24) weeks. In seven cases, thoracic masses were described as extralobar receiving the blood supply from Thoracic Descending Aorta (TDA) and Abdominal Descending Aorta (ADA) in five and two cases respectively. In the remaining three cases thoracic masses were described as intralobar receiving the blood supply from TDA. In three cases BPS was associated with major mediastinal shift. There were no cases of pleural effusion or hydrops in this group. In one case there was mild polyhydramnios. In the prenatal ultrasound examinations, no additional abnormalities were detected and in all cases the fetal karyotype was normal (Figure 1).

Figure 1: Ryc 1. Massive pleural effusion due to BPS.
The partial regression, no change and progression of the BPS volume in the subsequent ultrasound scans was found in six, three and one case respectively. The median gestational age at birth was 38.5 (37–40) weeks and in all cases the 5-minute Apgar score was >8. Postnatal excision of the lesion was carried out in seven of the neonates at 7–12 months of life. Three newborns which are now >12 months did not require surgery. All 10 survivors had follow-up for at least 12 months and they are healthy with no neurological disabilities.
Fetuses managed with intrauterine therapy
The median gestational age at the time of intervention was 22 (range 20 – 25) weeks. In all cases the thoracic mass of extralobar type with blood supply from thoracic descending aorta was associated with major mediastinal shift. In all cases there was associated hydrops and polyhydramnios. No additional abnormalities were detected and in all cases the fetal karyotype was normal.
In the group of fetuses treated with thoraco-amniotic shunt, the procedure was conducted successfully in all cases and resulted in the resolution of hydrops within one week of shunt insertion. However, in one case the procedure was repeated because of the dislocation of the initial shunt into the amniotic cavity and hydrops recurrence. No significant change in the volume of BPS was found in subsequent ultrasound scans. Two fetuses were delivered vaginally before 37 weeks due to preterm rupture of membranes. One fetus was born via elective cesarean section at 38 weeks (breech presentation).
In the group of fetuses treated with laser coagulation of the feeding vessel, the procedure was uncomplicated in all cases and in four out of five fetuses resulted in the resolution of the hydrops within one week. In one case, laser coagulation successfully ceased the blood supply, however hydrops has not resolved within 10 days after the procedure and the patient was offered another intervention using thoraco-amniotic shunt which was successfully inserted at 22 weeks’ gestation leading to complete resolution of hydrops. In the subsequent ultrasound scans a significant regression of the thoracic mass was found in all cases. All fetuses were born at term; three did not require surgery and two had sequestrectomy at the age of 6 and 8 months due to recurrent pulmonary infections.
The literature search identified a total of 228 fetuses managed expectantly and 82 fetuses managed with minimally-invasive methods (thoraco-amniotic shunt, laser coagulation of the feeding vessel, alcohol injection) between 1986 and 2019 (Table 3 and 4). In the combined data from the previous and the current study, the survival rate was 97.1% in the expectant management group (98.5% non-hydropic fetuses) and 93.2% in the invasive management group (100% hydropic fetuses). The need for postnatal sequestrectomy was lower in the group of fetuses treated with laser coagulation of the feeding vessel 8/25 (32%) in comparison to fetuses treated by thoraco-amniotic shunts 18/22 (81%). In the group treated with laser the rate of preterm birth (PTD) before 37 weeks was 16.7% and in the group treated with thoraco-amniotic shunt the rate of PTD was 63.6% (Table 5 and 6).
Author
n
GA at diagnosis
Hydrops
Fetal / Neonatal death
Survival
GA birth
Surgery
[33]
2
25 - 26
-
-
2 (100%)
37 - 38
0 / lost to follow-up
[34]
2
18
-
-
2 (100%)
39 – 40
0
[35]
3
25 - 34
+ (2) ascites (1)
-
3 (100%)
30 - 35
3
[36]
3
25 - 34
+ (3)
-
3 (100%)
30 - 36
3
[1]
37
18 - 36
+ (1) ascites (1)
1 (neonatal)
36
no data
7
[11]
8
20 - 33
-
-
8 (100%)
37 - 40
8
[37]
13
24
+ (2)
2 (neonatal)
11 (84%)
no data
11
[38-40]
2
19 - 20
-
-
2 (100%)
38 - 40
1
[41]
4
19 - 29
-
-
4 (100%)
no data
2
[42]
3
21 - 33
-
1 (neonatal)
2 (77%)
no data
3
[23]
6
19 - 35
-
-
6 (100%)
37 - 42
5
[18]
29
20 - 27
-
-
29 (100%)
34 - 39
16
[19]
7
22
-
7 (100%)
39
no data
[43]
7
[2nd – 3rd]
7 (100%)
>35
4
[44]
2
20 - 22
-
2 (100%)
34 - 38
0
[20]
100
19 - 37
5
100 (100%)
28 - 41
76
Present series
10
23 - 26
-
10 (100%)
37 – 40
7
Total
238
15/238 [6.3%]
4/238 [1.7%]
234/238 [98.3%]
146/220 [66.45]
Table 3: Data of fetuses with BPS managed expectantly.
Author
GA at interv.
Method of treatment
Indication
Effect
Outcome
GA at birth
Reason for delivery
<37 w
Surgery
Hydrothorax
Hydrops
Other
[7]
24
Thoracocentesis + Thoraco-amniotic shunt
Yes
yes
-
Reaccumultion
NND
29
Fetal distress
yes
[8]
32
Thoracocentesis + Thoraco-amniotic shunt
Yes
yes
-
Resolved
LB
34
PROM
yes
[9]
27
Thoraco-amniotic shunt
yes
-
-
Resolved
LB
36
No data
yes
[10]
30
Thoraco-amniotic shunt
Yes
yes
-
Resolved
LB
38
-
yes
[1]
29
Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
33 - 35
No data
yes
[1]
30
Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
33 - 35
No data
yes
[11]
30
Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
38
-
yes
[12]
23
Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
33
PROM
no data
[12]
30
Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
33
PROM
no data
[13]
34
Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
36
PROM
no
[14]
19-36
Thoraco-amniotic shunt
yes
yes
-
No data
LB
28-40
No data
no data
[15]
30
Thoracocentesis + Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
35
Maternal PE
yes
[15]
28
Thoracocentesis + Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
33
Maternal PE
yes
[15]
30
Thoracocentesis + Thoraco-amniotic shunt
yes
yes
-
Resolved
LB
35
PROM
yes
[16]
27
Thoraco-amniotic shunt
yes
-
-
Reaccumulated
LB
37
-
yes
[17]
27
Thoracocentesis + Thoraco-amniotic shunt
yes
-
-
Resolved
LB
35
No data
yes
[17]
30
Thoracocentesis + Thoraco-amniotic shunt
yes
-
-
Resolved
LB
33
No data
yes
[17]
30
Thoracocentesis + Thoraco-amniotic shunt
yes
-
-
Resolved
LB
35
No data
yes
[18]
25 - 29
Thoraco-amniotic shunt
yes
yes*
-
-
IUD
-
-
-
[18]
25 - 29
Thoraco-amniotic shunt
yes
yes*
-
Resolved
LB
30 - 37
No data
yes
[18]
25 - 29
Thoraco-amniotic shunt
yes
yes*
-
Resolved
LB
30 - 37
No data
yes
[18]
25 - 29
Thoraco-amniotic shunt
yes
yes*
-
Resolved
LB
30 - 37
No data
yes
[18]
25 - 29
Thoraco-amniotic shunt
yes
-
-
Resolved
LB
30 - 37
No data
yes
[18]
25 - 29
Thoraco-amniotic shunt
yes
-
-
Resolved
LB
30 - 37
No data
yes
[18]
25 - 29
Thoraco-amniotic shunt
yes
-
-
Resolved
LB
30 - 37
No data
no
[19]
29
Thoracocentesis + Thoraco-amniotic shunt
yes
yes
Resolved
LB
33
No data
no
[19]
30
Thoracocentesis + Thoraco-amniotic shunt
yes
yes
Resolved
NND
36
No data
no
[20]
23
Thoraco-amniotic shunt
yes
yes
Resolved
LB
35
No data
no
[20]
27
Thoraco-amniotic shunt
yes
yes
Resolved
LB
33
No data
yes
Present series
21
Thoraco-amniotic shunt
yes
Resolved
LB
39
-
yes
Present series
23
Thoraco-amniotic shunt
yes
Resolved
LB
38
-
yes
Present series
20
Thoraco-amniotic shunt
yes
Resolved
LB
38
-
yes
30/32 [100%]
21/32 [65.6%]
30/32 [93.7%]
23/28 [82.1%]
*in the series of Mallman et al. four out of seven fetuses treated with TAS were hydropic and five out of six surviving neonates required sequestrectomy. An individual analysis of each case was not presented in the study.
Table 4: Data of fetuses with BPS treated with thoraco-amniotic shunt.
Author
GA at interv.
Method of treatment
Indication
Effect
Outcome
GA at birth
Reason for delivery
<37 w
Surgery
Hydrothorax
Hydrops
Other
[21]
23
Laser coagulation
yes
yes
-
Resolved
LB
39
-
no
[22]
29
Laser coagulation
yes
yes
-
Resolved
LB
38
-
yes
[23]
31
Laser coagulation
yes
-
-
Resolved
LB
38
-
yes
[23]
30
Laser coagulation
yes
-
-
Resolved
LB
38
-
yes
[23]
32
Laser coagulation
yes
-
-
Resolved
LB
34
-
no
[23]
27
Laser coagulation
yes
-
-
Resolved
LB
41
-
no
[23]
24
Laser coagulation
yes
-
-
Resolved
LB
40
-
no
[23]
31
Laser coagulation
yes
-
-
Resolved
LB
34
No data
yes
[23]
23
Laser coagulation
yes
-
-
Resolved
LB
35
No data
yes
[23]
28
Laser coagulation
yes
-
-
Resolved
LB
39
-
yes
[24]
23
Laser coagulation
yes
yes
-
Resolved
LB
41
-
no
[25]
24
Laser coagulation
yes
yes
-
Resolved
LB
36
no data
No data
[25]
28
Laser coagulation
yes
yes
-
Resolved
LB
39
no data
No data
[25]
29
Laser coagulation
yes
yes
-
No change
IUD
30
IUD
No data
[18]
24 - 31
Laser coagulation
yes
yes*
-
Resolved
LB
38 - 40
-
yes
[18]
24 - 31
Laser coagulation
yes
-
-
Resolved
LB
38 - 40
-
no
[18]
24 - 31
Laser coagulation
yes
-
-
Resolved
LB
38 - 40
-
no
[18]
24 - 31
Laser coagulation
yes
-
-
Resolved
LB
38 - 40
-
no
[18]
24 - 31
Laser coagulation
yes
-
-
Resolved
LB
38 - 40
-
no
[26,27]
28
Laser coagulation
yes
yes
-
Resolved
LB
38
-
no
[26,27]
28
Laser coagulation
yes
yes
-
Resolved
LB
38
-
no
[26,27]
20
Laser coagulation
yes
-
-
Resolved
LB
37
-
no
[26,27]
31
Laser coagulation
yes
-
-
Resolved
LB
39
no
[26,27]
28
Laser coagulation
yes
yes
-
Resolved
LB
39
no
[26,27]
28
Laser coagulation
yes
-
-
Resolved
LB
35
No data
no
[26,27]
24
Laser coagulation
yes
-
-
Resolved
LB
38
no
[26,27]
23
Laser coagulation
yes
-
-
Resolved
LB
40
-
no
[26,27]
24
Laser coagulation
yes
-
-
Resolved
LB
38
-
no
[26,27]
24
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[26,27]
30
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[26,27]
27
Laser coagulation
yes
-
-
Resolved
LB
38
-
no
[26,27]
25
Laser coagulation
yes
yes
-
Resolved
LB
38
-
no
[26,27]
27
Laser coagulation
yes
-
-
Resolved
LB
37
-
no
Table 5: Data of fetuses with BPS treated with laser coagulation of the feeding vessel.
[26,27]
27
Laser coagulation
yes
-
-
Resolved
LB
37
-
no
[26,27]
30
Laser coagulation
yes
-
-
Resolved
LB
40
-
no
[28]
22
Laser coagulation
-
-
Ascites
Resolved
LB
39
-
no
[28]
27
Laser coagulation
yes
yes
-
Resolved
LB
41
-
no
[29]
31, 32
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[29]
32, 33
Laser coagulation
yes
-
-
Resolved
LB
41
-
yes
[29]
34
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[29]
25
Laser coagulation
yes
-
-
Resolved
LB
42
-
no
[29]
32
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[29]
32
Laser coagulation
yes
-
-
Resolved
LB
42
-
yes
[29]
28, 30
Laser coagulation + Thoracocentesis
yes
-
-
Resolved
LB
30
PPROM, choriomnionitis
yes
[29]
31
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[29]
30
Laser coagulation
yes
-
-
Resolved
LB
39
-
no
[29]
31, 31
Laser coagulation
yes
-
-
Resolved
LB
41
-
no
[29]
33, 34
Laser coagulation
yes
-
-
Resolved
LB
38
-
no
Present series
Laser coagulation
yes
yes
-
Resolved
LB
Present series
Laser coagulation
yes
-
-
Resolved
LB
Present series
Laser coagulation
yes
-
-
Resolved
LB
Present series
Laser coagulation
yes
-
-
Resolved
LB
Total
51 fetuses
50/51 [98.0%]
Table 5 of 1:
Author
GA at interv.
Method of treatment
Indication
Effect
Outcome
GA at birth
Reason for delivery
<37 w
Surgery
Hydrothorax
Hydrops
Other
[30]
27
Alcohol injection + thoracoamniotic shunt
yes
yes
-
Resolved
LB
40
-
no
[31]
26
Polidocanol injection
yes
yes
-
Resolved
LB
38
-
yes
[31]
26
Polidocanol injection
no
no
Mediastinal shift
-
LB
38
-
no
[31]
24
Polidocanol injection
-
-
Ascites
Resolved
NND
38
-
yes
[32]
30
Thoracoamniotic shunt
+ laser coagulation
+ thoracocentesis
yes
-
-
Resolved
LB
No data
yes
[32]
31, 31
Thoracoamniotic shunt
+ laser coagulation
+ thoracocentesis
yes
-
-
Resolved
LB
No data
yes
[29]
25, 26
Thoracoamniotic shunt
+ laser coagulation
yes
-
-
Resolved
LB
40
no
Present series
Table 6: Data of fetuses with BPS treated with intratumor injection of sclerosant or combined treatment.
Discussion
Main findings of this study
The data of this study and previous reports demonstrate the favourable prognosis in cases of BPS without associated hydrothorax and/or hydrops. It also shows the efficiency of intrauterine therapy in treatment of BPS with associated hydrops. Moreover, laser coagulation of the feeding vessel is likely to contribute to the reduction of the lesion’s volume and as a result the need for postnatal surgery is diminished.
In the expectant management group, lack of progression of the lesion’s volume was stated in the vast majority of cases (90%). All infants were born in a good overall condition and did not require any treatment in the first month of life. In our centre the inclusion criteria for fetal intervention in case of BPS are either hydrops or severe hydrothorax. Additional abnormalities were stated in 22% of cases. These cases were excluded from further analysis.
In all fetuses with hydrops the intrauterine management using thoraco-amniotic shunt or laser coagulation of the feeding vessel resulted in resolution of hydrops. There were no cases of therapy complications. Additionally, in one of the 3 fetuses treated with thoraco-amniotic shunt there was a need for reinsertion because the first one was dislodged. The intrauterine management using thoracoamniotic shunt resulted in resolution of fetal hydrops without an impact on the lesion’s size.
Limitations of the study
The major limitations of the study relate its retrospective design, the small size of the group and lack of controls. However, these limitations result from the rarity of the condition.
Comparison of the findings with previous studies in the literature
Thoraco-amniotic shunt insertion in a hydropic fetus with brochopulmonary sequestration was first described by Weiner et al. [7]. Treatment resulted in resolution of hydrops, however the fetus was delivered prematurely. Another approach to the treatment of BPS by interstitial laser was first reported by Oepkes et al. in a fetus at 23 weeks gestation presenting with a large solid mass and hydops. Cavoretto et al. were first to publish a series of cases treated with laser ablation and proved the feasibility and safety of this technique. Since than a number of studies have been published. In a large series by Mallman et al. [16], that compared thoraco-amniotic shunting and interstitial laser in BPS with pleural effusion, the survival rate was 85.7% (6/7) in the group treated with thoraco-amniotic shunting and 100% (5/5) in the group treated with laser coagulation. However, the authors found that laser ablation was associated with significantly better perinatal outcome compared to the group treated with thoraco-amniotic shunting: gestational age at delivery was higher (median age, 39.1 (range 38.0-40.0) vs 37.2 (range 30.3-37.4)) and the need for postnatal sequestercomy was lower (20% vs 83.3%). In this extended summary, including our data, the survival rate in the invasive management group was 97.1%: 93.7% (30/32) in the group treated with thoraco-amniotic shunting and 98% (50/51) in the group treated with laser coagulation. However, survival does not always mean success defined as a term birth of an neurologically healthy child. The major risk factor of neurological impairment is still preterm birth (PTD). In the group treated with laser the rate of PTD before 37 weeks was 16.7% and in the group treated with thoracoamniotic shunting the rate of PTD was 63.6%. Also, the need for postnatal sequestrectomy was lower in the group of fetuses treated with laser coagulation 8/25 (32%) in comparison to fetuses treated by thoraco-amniotic shunts 18/22 (81%).
Implications for clinical practice
The role of intrauterine therapy in case of hydropic fetuses with bronchopulmonary sequestration is well accepted. Several case reports suggest that fetuses with BPS and hydrops managed expectantly have a very poor prognosis due to pulmonary hypoplasia [34,35]. Successful treatment with thoraco-amniotic shunt contributes to the improvement of fetal well-being and diminishes the likelihood of intrauterine demise as well as neonatal death due to pulmonary hypoplasia. However, this kind of treatment is strictly symptomatic. Laser coagulation of the feeding vessel is likely to not only diminish the signs of hydrops but also to be the definitive treatment by ceasing the blood supply to the pathological lung mass. It should be therefore considered as the most appropriate method of treatment in hydropic fetuses with BPS.
References
- Adzick NS, Harrison MR, Crombleholme TM, Flake AW, Howell LJ. Fetal lung lesions: management and outcome. Am J Obstet Gynecol. 1998; 179: 884–889.
- Hernanz-Schulman M, Stein SM, Neblett WW, Atkinson JB, Kirchner SG, Heller RM, et al. Pulmonary sequestration: diagnosis with color Doppler sonography and a new theory of associated hydrothorax. Radiology. 1991; 180: 817–821.
- Jones DA, Vill MD, Izquierdo LA. Lung sequestration, extralobar intrathoracic. TheFetus. Net.1992.
- Anandakumar C, Biswas A, Chua TM, Choolani M, Chia D, Wong YC, et al. Direct intrauterine fetal therapy in a case of bronchopulmonary sequestration associated with nonimmune hydrops fetalis. Ultrasound Obstet Gynecol. 1999; 13: 263–265.
- Morville P, Malo-Ferjani L, Graesslin O, Bory JP, Harika G. Physiopathology hypotheses and treatment of pulmonary sequestration. Am J Perinatol. 2003; 20: 87–89.
- Pumberger W, Hormann M, Deutinger J, Bernaschek G, Bistricky E, Horcher E. Longitudinal observation of antenatally detected congenital lung malformations (CLM): natural history, clinical outcome and long-term followup. Eur J Cardiothorac Surg. 2003; 24: 703–711.
- Weiner C, Varner M, Pringle K, Hein H, Williamson R, Smith WL. Antenatal diagnosis and palliative treatment of nonimmune hydrops fetalis secondary to pulmonary extralo- bar sequestration. Obstet Gynecol. 1986; 68: 275–280.
- Slotnick RN, McGahan J, Milio L, Schwartz M, Ablin D. Antenatal diagnosis and treatment of fetal bronchopulmonary sequestration. Fetal Diagn Ther. 1990; 5: 33–39.
- Hernanz-Schulman M, Stein SM, Neblett WW, Atkinson JB, Kirchner SG, Heller RM, et al. Pul- monary sequestration: diagnosis with color Doppler sonography and a new theory of associated hydrothorax. Radiology. 1991; 180: 817–821.
- Favre R, Bettahar K, Christmann D, Becmeur F. Antenatal diagnosis and treatment of fetal hydrops secondary to pulmonary extralobar sequestration. Ultrasound Obstet Gynecol. 1994; 4: 335–338.
- Becmeur F, Horta-Jeraud P, Donato L, Sauvage P. Pulmonary sequestration: prenatal ultrasound diagnosis, treatment and outcome. J Pediatr Surg. 1998; 33: 492–496.
- Lopoo JB, Goldstein RB, Lipshutz GS, Goldberg JD, Harrison MR, Albanese CT. Fetal pulmonary sequestration: a favorable congenital lung lesion. Obstet Gynecol 1999; 94: 567–571.
- Salomon LJ, Audibert F, Dommergues M, Vial M, Frydman R. Fetal thoracoamniotic shunting as the only treatment for pulmonary sequestration with hydrops: favorable long- term outcome without postnatal surgery. Ultrasound Obstet Gynecol. 2003; 21: 299–301.
- Picone O, Benachi A, Mandelbrot L, Ruano R, Dumez Y, Dommergues M. Thoracoamniotic shunting for fetal pleural effusions with hydrops. Am J Obstet Gynecol. 2004; 191: 2047-2050.
- Hayashi S, Sago H, Kitano Y, Kuroda T, Honna T, Nakamura T, et al. Fetal pleuroamniotic shunting for bronchopulmonary sequestration with hydrops. Ultrasound Obstet Gynecol. 2006; 28: 963–967.
- Odaka A, Honda N, Baba K, Tanimizu T, Takahashi S, Ohno Y, et al. Pulmonary sequestration. J Pediatr Surg. 2006; 41: 2096–2097.
- Kitano Y, Matsuoka K, Honna T, Kuroda T, Morikawa N, Hayashi S, et al. Venous arterialization in extralobar pulmonary sequestration associated with fetal hydrops. J Pediatr Surg. 2006; 41: 490-494.
- Mallmann MR, Geipel A, Bludau M, Berg C. Bronchopulmonary sequestration with massive pleural effusion: pleuroamniotic shunting vs intrafetal vascular laser ablation. Ultrasound Obstet Gynecol. 2014; 44: 441- 446.
- Stoiber B, Moehrlen U, Kurmanavicius J, Meuli M, Ochsenbein N. Congenital Lung Lesion: prenatal course, therapy and perinatal outcome. Ultraschall in Med. 2015.
- Riley JS, Urwin JW, Oliver ER, Coleman BG, Khalek N, Moldenhauer JS, et al. Prenatal growth characteristics and pre/postnatal management of bronchopulmonary sequestrations. J Pediatr Surg. 2018; 53: 265-269.
- Oepkes D, Devlieger R, Lopriore E, Klumpfer FJCM. Successful ultrasoundguided laser treatment of fetal hydrops caused by pulmonary sequestration. Ultrasound Obstet Gynecol. 2007; 29: 457–459.
- Ruano R, de A Pimenta EJ, Marques da Silva M, Maksoud JG, Zugaib M. Percutaneous intrauterine laser ablation of the abnormal vessel in pulmonary sequestration with hydrops at 29 weeks’ gestation. J Ultrasound Med. 2007; 26: 1235–1241.
- Cavoretto P, Molina F, Poggi S, Davenport M, Nicolaides KH. Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet Gynecol. 2008; 32: 769-683.
- Witlox RS, Lopriore E, Walther FJ, Rikkers-Mutsaerts ER, Klumper FJ, Oepkes D. Single-needle laser treatment with drainage of hydrothorax in fetal bronchopulmonary sequestration with hydrops. Ultrasound Obstet Gynecol. 2009; 34: 355–357.
- Ruano R, da Silva MM , Assunção Salustiano EM , Kilby MD, Tannuri U, Zugaib M. Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenatal Diagnosis. 2012; 32: 1–6.
- Cruz-Martinez R, Mendez A, Duenas-Riano J, Rebolledo-Fernandez C. Fetal laser surgery prevents fetal deaths and avoids the need for neonatal sequestrectomy in cases with bronchopulmonary sequestration. Ultrasound Obstet Gynecol. 2015; 24: 627-632.
- Cruz-Martínez R, Nieto-Castro B, Martínez-Rodríguez M, Gámez-Varela A, Ahumada-Angulo E, Luna-García J, et al. Thoracic Changes after Full Laser Ablation of the Feeding Artery in Fetuses with Bronchopulmonary Sequestration. Fetal Diagn Ther. 2018; 44: 166-172.
- Kosinski P, Tavares de Sousa M, Wielgos M, Hecher K. Intrauterine Ultrasound-Guided Laser Coagulation of the Feeding Artery in Fetal Bronchopulmonary Sequestration. Ultraschall Med. 2017; 38: 583-586.
- Gottschalk I, Strizek B, Mallmann MR, Müller A, Geipel A, Gembruch U, et al. Outcome of Bronchopulmonary Sequestration with Massive Pleural Effusion after Intrafetal Vascular Laser Ablation. Fetal Diagn Ther. 2018; 44: 149-155.
- Nicolini U, Cerri V, Groli C, Poblete A, Mauro F. A new approach to prenatal treatment of extralobar pulmonary sequestration. Prenat Diagn. 2000; 20: 758–760.
- Bermudez C, Perez-Wulff J, Bufalino G, Sosa C, Gomez L, Quintero RA. Percutaneous ultrasound-guided sclerotherapy for complicated fetal intralobar bronchopulmonary sequestra- tion. Ultrasound Obstet Gynecol. 2007; 29: 586–589.
- Rammos KS, Foroulis CN, Rammos CK, Andreou A. Prenatal interventional and postnatal surgical therapy of extralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg. 2010; 10: 634–635.
- Langer B, Donato L, Riethmuller C, Becmeur F, Dreyfus M, Favre R, et al. Spontaneous regression of fetal pul- monary sequestration. Ultrasound Obstet Gynecol. 1995; 6: 33–39.
- Abuhamad AZ, Bass T, Katz ME, Heyl PS. Familial recurrence of pulmonary sequestration. Obstet Gynecol. 1996; 87: 843-845.
- da Silva OP, Ramanan R, Romano W, Bocking A, Evans M. Nonimmune hydrops fetalis, pulmonary sequestration, and favorable neonatal outcome. Obstet Gynecol. 1996; 88: 681-683.
- Evans MG. Hydrops fetalis and pulmonary sequestration. J Pediatr Surg. 1996; 31: 761-764.
- Bratu I, Flageole H, Chen MF, Di Lorenzo M, Yazbeck S, Laberge JM. The multiple facets of pulmonary sequestration. J Pediatr Surg. 2001; 36: 784- 790.
- Chen JSC, Walford N, Yan YL, Ong CL, Yeo GSH. Foetal intralobar lung sequestration: antenatal diagnosis and management. Singapore Med J. 2003; 44: 630-634.
- Chen CP, Liu YP, Lin SP, Sheu JC, Hsu CY, Chang TY, et al. Prenatal magnetic resonance imaging demonstration of the systemic feeding artery of a pulmonary sequestration associated with in utero regression. Prenat Diagn. 2005; 25: 715-726.
- Chen CP, Liu YP, Hsu CY, Lin SP, Wang W. Prenatal sonography and magnetic resonance imaging of pulmonary seques- tration associated with a gastric duplication cyst. Prenat Diagn. 2006; 26: 489–491.
- Illanes S, Hunter A, Evans M, Cusick E, Soothill P. Prenatal diagnosis of echogenic lung: evolution and outcome. Ultrasound Obstet Gynecol. 2005; 26: 145–149.
- Ruano R, Benachi A, Aubry MC, Revillon Y, Emond S, Dumez Y, et al. Prenatal diagnosis of pulmonary sequestration using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol. 2005; 25: 128-133.
- Nunes C, Pereira I, Araujo C, Mendes Graca L. Fetal bronchopulmonary malformations. The Journal of Maternal-Fetal & Neonat Med. 2015; 28: 1996- 2000.
- Pinto RM, Araujo Junior E, Costa Augusto L, Herlanio Carvalho F. Spontaneous regression of intralobar pulmonary sequestration during pregnancy: report of two cases through relationships between mass and fetal biometry and review of the literature. The Journal of Maternal-Fetal & Neonat Med. 2016; 29: 1720-1724.