
Research Article
Austin J Radiol. 2021; 8(10): 1164.
Development of a Computerized, Evidence-Based Decision Support System for Computed Tomography Assessment in Acute Aortic Syndrome in the Emergency Department
Lumbreras-Fernández B1*, Vicente Bártulos A2, Fernandez-Felix BM3, Corres González J4, Zamora J3,5 and Muriel A6
1Radiology Department, Hospital Universitario Ramón y Cajal, Madrid, Spain
2Emergency Radiology Department, Hospital Universitario Ramón y Cajal, Madrid, Spain
3Clinical Biostatistics Unit, Hospital Universitario Ramon y Cajal (IRYCIS), CIBER Epidemiology and Public Health (CIBERESP), Madrid, Spain
4Emergency Department, Hospital Universitario Ramón y Cajal, Madrid, Spain
5Institute of Metabolism and Systems Research, University of Birmingham, England
6Clinical Biostatistic Unit, Hospital Universitario Ramón y Cajal, IRYCIS, CIBER Epidemiology and Public Health (CIBERESP), Nursing Department, Universidad de Alcalá, Madrid, Spain
*Corresponding author: Lumbreras-Fernández B, Radiology Department, Hospital Infanta Sofía, San Sebastián de los Reyes, 28703 Madrid, Spain
Received: August 31, 2021; Accepted: October 06, 2021; Published: October 13, 2021
Abstract
Purpose: To develop a computerized algorithm, or Clinical Decision Support System (CDSS), for managing and requesting imaging in the emergency department, specifically Computerized Tomography Angiography (CTA) of the aorta, when there is suspicion of AAS, and to determine the effect of implementing this system. To determine the factors associated with a positive radiological diagnosis that improve the predictive capacity of CTA findings.
Methods: After developing and implementing an evidence-based algorithm, we studied suspected cases of AAS. Chi-squared test was used to analyze the association between the variables included in the algorithm and radiological diagnosis, with three categories: no relevant findings, positive for AAS, and alternative diagnoses.
Results: 130 requests were identified; 19 (14.6%) had AAS and 34 (26.2%) had a different acute pathology. Of the 19 with AAS, 15 had been stratified as high risk and 4 as intermediate risk. The probability of AAS was 3.4 times higher in patients with known AA (p = 0.021, 95% CI 1.2-9.6) and 5.1 times higher in patients with a new aortic regurgitation murmur (p = 0.019, 95% CI 1.3-20.1). The probability of having an alternative severe acute pathology was 3.2 times higher in patients with hypotension or shock (p = 0.02, 95% CI 1.2-8.5).
Conclusion: The use of a CDSS in the emergency department can help optimize AAS diagnosis. In our hospital it improved AAS management and the diagnostic yield of CTA.
Keywords: Acute aortic syndrome; Chest pain; Thoracic pain; Algorithm; Aortic CT angiography; Clinical decision support system (CDSS)
Abbreviations
AAS: Acute Aortic Syndrome; ACCF/AHA: American College of Cardiology Foundation/American Heart Association; ACR: American College of Radiology; AA: Aortic Aneurysm; CDSS: Clinical Decision Support Systems; CTA-A: Computed Tomography Angiography of the Aorta; ESC: European Society of Cardiology; PTE: Pulmonary Thromboembolism
Introduction
Acute Aortic Syndrome (AAS) has an estimated incidence of 2-3.5/100,000 population/year [1].
The classical presentation is of sudden onset of intense chest, abdominal, or back pain, described as sharp, piercing, tearing, or stabbing. Although pain is the most reported symptom, there is great variety in clinical presentation [2]. There are also several processes that can mimic AAS, including acute coronary syndrome, pleuropulmonary, gastrointestinal, or musculoskeletal pathology, hypotension, and visceral or limb ischemia [3].
This varied presentation along with the lack of specific biomarkers make AAS difficult to diagnose. Due to its rapid progression and high mortality (40% immediate mortality and 1-2% per hour from the onset of symptoms), tools for a rapid, accurate diagnosis are needed [1,4,5].
Due to the prognostic implications of diagnostic error or delay, algorithms or clinical decision support systems (CDSS) are essential to guide the clinician in their diagnostic approach.
The main objectives of this study were:
• To develop a CDSS to improve the appropriateness of Computed Tomography Angiography (CTA) of the aorta when AAS is suspected in the emergency department and to determine the effect of its implementation.
• To identify the risk factors (past medical history, presentation, examination findings) associated with a positive diagnosis of AAS on CTA and that could help in developing a clinical prediction rule.
Materials and Methods
This study forms part of the multicenter project MAPAC-imagen (Mejora de la Adecuación de la Práctica Asistencial y Clínica, meaning Improvement of Appropriateness of Health Care and Clinical Practice) funded by the ISCIII as part of their Acción Estratégica en Salud (Strategic Health Action) between 2013 and 2016. The project was approved by the hospital ethics committee.
The study was conducted in the following phases:
Phase 1: Development of the algorithm for radiological management in cases of suspected AAS
Literature review: The databases Best Practice, Dynamed, Up to Date, Ovid, MEDLINE and EMBASE were consulted, as well as repositories of clinical practice guidelines, ACR guidelines (ACR appropriateness criteria), guidelines from the ACCF/AHA and the ESC, to identify relevant documents on the diagnostic management and risk factors for AAS.
For this systematic search we combined search terms associated with the disease (acute aortic syndrome, aortic dissection, acute intramural hematoma, penetrating aortic ulcer, periaortic hematoma, unstable aneurysm), the reason for attendance (acute chest pain, thoracic pain, chest pain, sudden onset excruciating anterior or interscapular), crossing them with terms for the imaging technique and synonyms (aortic CT angiography, CT angiography, contrast enhanced CT), and the study setting (emergency). To restrict the search we used methodological filters for clinical prediction rules including Haynes Broad Filter (HBF) and Teljeur/Murphy Inclusion Filter 26 items (TMIF-26) and exclusion filter (TMEF).
Development of the algorithm, consensus and implementation: The documents identified in the search were screened, and those considered most relevant as a source of evidence were selected to create the decision support algorithm on the use of CTA for diagnosis of AAS. After analyzing the selected literature we created a narrative synthesis, and the information was used to design an algorithm that took into account the risk factors analyzed in these studies. This algorithm was discussed and consensus opinion sought in an inperson meeting of experts using the Delphi panel technique to assess the appropriateness of the factors included and a second virtual round to reach consensus on factors which had not been agreed upon in the in-person round.
The final algorithm was integrated in the electronic medical record system of our hospital, so that when CTA of the aorta was requested in patients with suspicion of AAS, it generated a pop-up window with questions prompting selection of risk factors. Based on these, it stratified the degree of suspicion of AAS, and the system would then indicate whether or not CTA of the aorta would be appropriate.
Phase 2: Analysis of the outcomes of implementation
Design:
Impact of implementation of the algorithm: This was a beforeand- after study (pre- and post-implementation of the CDSS), of 6 months’ duration for each period, in which all requests for CTA aorta for suspected AAS were collected. We evaluated the number of requests for CTA for suspected AAS and the diagnostic yield of these (normal study, findings of AAS, or other unrelated findings).
Exploration of risk factors associated with radiological findings: All cases of suspected AAS in the 27 successive months after implementation of the algorithm (March 2016 to June 2018) were studied. We evaluated the association between the factors included in the CDSS and the radiological findings on CTA.
Statistical analysis
Univariate multinomial regression models were used to evaluate the association between the risk factors included in the algorithm and the radiological findings, with three categories: normal study or irrelevant/nonpathological findings, study diagnostic of AAS, or study with findings of other acute pathologies different from AAS. No multivariate models were used due to the low frequency of positive findings.
P-values <0.05 were considered indicative of statistical significance.
For statistical analysis the program STATA v.15.1 (StataCorp LLC, 4905 Lakeway Drive, College Station, Texas, USA) was used.
Results
Literature review
The literature search identified 573 studies; 6 of these were excluded as duplicates and 550 as the articles did not meet the criteria (e.g. case series, primary studies, studies not in English or Spanish, or in a pediatric population). Ultimately, the analysis and qualitative synthesis included 17 studies that were used to develop the decision support algorithm. These studies were mainly clinical practice guidelines [1,4], imaging appropriateness guidelines [5-7], systematic reviews [8-14] and metaanalysis [15] and other studies that evaluated risk factors for AAS [16,17]. No clinical prediction rules were identified. The screening and selection process is shown in Figure 1.

Figure 1: Literature search process.
Development and application of the algorithm
Based on the algorithm proposed by the ACCF/AHA [1] we created a modified decision support algorithm. This algorithm was discussed among the panel of experts until a consensus was reached. The outcome of the consensus is shown in Figure 2.
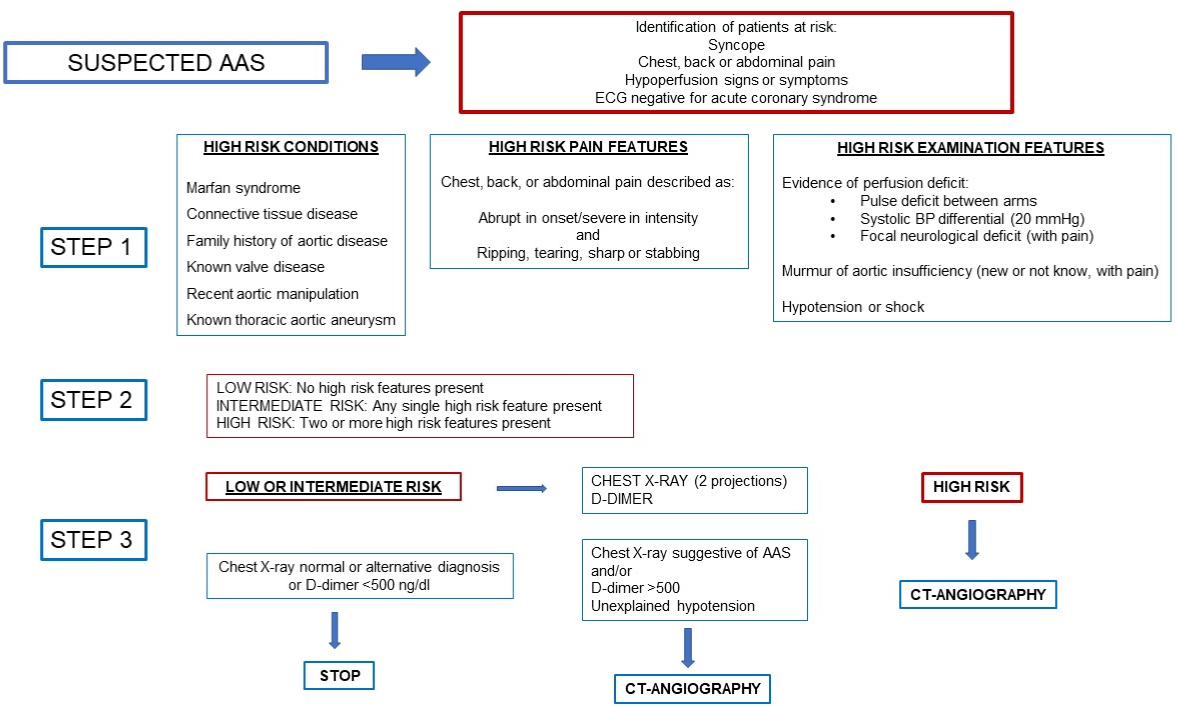
Figure 2: Algorithm for AAS risk stratification and diagnostic management.
Step 1: Scoring based on the presence of risk factors. Each existing condition scores one point. Abrupt-onset, severe pain with any of the classical characteristics
described (it need not meet all descriptive criteria) scores 1 point. Any sign of perfusion deficit, new-onset aortic regurgitation murmur or the presence of hypotension
or shock each score 1 point, independently.
Step 2: Risk stratification. Score 0: low risk. Score 1: Intermediate risk. Score 2 or more: high risk.
Step 3: Diagnostic approach. Low or intermediate risk: measure D-dimer (with cutoff of 500 ng/mL) and perform PA and lateral chest X-ray. If these suggest a
different diagnosis or are normal, CTA of the aorta is not indicated. If D-dimer is raised, chest X-ray suggests aortic pathology, or there is unexplained sustained
hypotension, CTA of the aorta should be performed. High risk: perform urgent CTA aorta as a first line.
The algorithm was designed for use in patients with clinical suspicion of AAS, identifying risk factors as indicated in Figure 2. It takes into account the presence, or not, of risk factors from the past medical history, clinical presentation, and examination findings.
Analysis of the outcomes of implementation
In the two periods analyzed, before and after implementation of the CDSS, a similar number of patients attended the emergency department (69081 and 72915, respectively). The total number of CTs requested was also similar (5081 and 5563, respectively). After implementation of the CDSS, the number of CTAs requested for suspicion of AAS doubled (10 vs. 21 requests). In the preimplementation period, the results of all the tests requested were negative for AAS; in the post-implantation period, AAS was diagnosed in one (5%). There was also an increase in acute pathologies mimicking AAS (5 cases, 24%).
In the post-implementation follow-up period (27 months), 130 CTA requests for suspected AAS were recorded; 19 of these (14.6%) were diagnostic of AAS and 34 (26.2%) of other acute pathology. In total, 53 patients (40.7%) benefitted from undergoing urgent CTA because it showed significant pathological findings.
The frequency of the different variables classified as risk factors is presented in Table 1, the most relevant ones being high-risk pain characteristics (83 patients, 63.8%) and a past history of AA (52.6%).
No Relevant Findings
AAS
Alternative Diagnosis
n=77
n=19
n=34
Marfan Syndrome
0 (0.0%)
0 (0.0%)
3 (8.8%)
Connective Tissue Disease
2 (2.6%)
1 (5.3%)
3 (8.8%)
Family History of Aortic Disease
5 (6.5%)
1 (5.3%)
4 (11.8%)
Known Valve Disease
26 (33.8%)
7 (36.8%)
5 (14.7%)
Recent Aortic Manipulation
6 (7.8%)
4 (21.1%)
3 (8.8%)
Aortic Aneurysm
19 (24.7%)
10 (52.6%)
6 (17.6%)
High-Risk Pain
48 (62.3%)
12 (63.2%)
23 (67.6%)
Perfusion Deficit
13 (16.9%)
4 (21.1%)
11 (32.4%)
Murmur of Aortic Regurgitation
5 (6.5%)
5 (26.3%)
5 (14.7%)
Hypotension or Shock
10 (13.0%)
5 (26.3%)
11 (32.4%)
Table 1: Absolute and Relative Frequencies of Each of the Positive Risk Factors Recorded on CTA Requests According to Radiological Diagnosis.
Regarding risk stratification, 10 patients (7.7%) were in the lowrisk group, 48 (36.9%) were in the intermediate-risk group and 72 (55.4%) were in the high-risk group.
Table 2 shows the number of CTAs that were positive for AAS, and for other serious acute pathologies, grouped according to pretest probability. Nineteen patients (14.6%) were diagnosed with AAS and 34 (26.2%) were diagnosed with other acute conditions.
Risk Category
No Relevant Findings
AAS
Alternative Diagnosis
Total
Low
7 (70%)
0 (0%)
3 (30%)
10
Intermediate
27 (56.3%)
4 (8.3%)
17 (35.4%)
48
High
43 (59.7%)
15 (20.8%)
14 (19.4%)
72
Total
77 (59.2%)
19 (14.6%)
34 (26.2%)
130
Table 2: Association Between Risk Groups by Pretest Probability of AAS; Absolute and Relative Frequencies of Findings on CTA Grouped into No Relevant Findings, Positive Findings for AAS and Findings Associated with Other Acute Processes.
The alternative radiological diagnoses in the patients with acute pathology other than AAS are presented in Table 3, with cardiac disease being the most prevalent (32.4%).
Percentage of Patients
Pulmonary Thromboembolism
3
8.80%
Pulmonary Disease
7
20.60%
Cardiac Disease
11
32.40%
Abdominal Disease
8
23.50%
Aortic Prosthesis Complication
3
8.80%
Bone Fracture (Ribs, Vertebrae)
2
5.90%
Total
34
100%
Table 3: Absolute and Relative Frequencies of Alternative Diagnoses Identified on CTA.
The univariate analysis of the association of the different radiological diagnoses with each of the factors included in the decision support algorithm is shown in Table 4. The probability of AAS was 3.4 times higher in patients with known AA (p = 0.021; 95% CI 1.2- 9.6) and 5.1 times higher in patients with a new murmur suggestive of aortic regurgitation (p = 0.019; 95% CI 1.3-20.1). The probability of having an alternative acute severe pathology was 3.2 times higher in patients with hypotension or shock (p = 0.02, 95% CI 1.2-8.5). This variable did not show an increased risk of AAS that reached statistical significance.
Univariate multinomial logistic regression
AAS RRR (95% CI)
Alternative Diagnosis RRR (95% CI)
Marfan Syndrome
Not estimable
Not estimable
Connective Tissue Disease
2.08 (0.18–24.26)
3.63 (0.58–22.79)
Family History of Aortic Disease
0.80 (0.09–7.28)
1.92 (0.48–7.65)
Known Valve Disease
1.14 (0.40–3.25)
0.34 (0.12–0.98)
Recent Aortic Manipulation
3.16 (0.79–12.57)
1.15 (0.27–4.88)
Aortic Aneurysm
3.39 (1.20–9.59)
0.65 (0.24–1.82)
High-Risk Pain
1.04 (0.37–2.93)
1.26 (0.54–2.97)
Perfusion Deficit
1.31 (0.37–4.60)
2.35 (0.93–5.99)
Murmur of Aortic Regurgitation
5.14 (1.31–20.15)
2.48 (0.67–9.22)
Hypotension or Shock
2.39 (0.71–8.09)
3.20 (1.20–8.53)
RRR: Relative Risk Ratio.
Table 4: Univariate Multinomial Logistic Regression Analysis of the Association of the Risk Factors from the Decision Support Algorithm with Findings of AAS or Other Unrelated Findings.
Chest X-ray was performed in 54 patients and provided an alternative diagnosis in 20 of them (37%). When analyzed with Chisquared test, the probability of having an alternative acute severe pathology was twice as high in patients with abnormalities on chest X-ray (p = 0.022).
Discussion
Prior to the use of algorithms, AAS was misdiagnosed in more than 30% of cases [6,7]. In 2010, the ACCF/AHA, along with other North American scientific societies and colleges published an algorithm for the diagnosis and management of AAS by stratification into three risk groups, using a scoring system based on the presence of defined risk factors: the Aortic Dissection Detection Risk Score (ADD-RS) [1]. In 2014 the ESC [4] and the ACR [5] published guidelines for the diagnosis and treatment of aortic disease and appropriate use of investigations.
These three guidelines recommend CTA aorta with cardiac synchronization as the technique of choice, especially in patients with a high pretest probability [5]. This also allows a triple rule-out of PTE and acute coronary syndrome. Plain chest X-ray can help to establish an alternative diagnosis in patients with low or intermediate risk [1]. In low-risk patients, the ESC allows rule-out of AAS with a chest X-ray not suggestive of AAS and negative D-dimer (less than 500ng/dL) [4].
The high prevalence of cardiac disease mimicking AAS, confirmed in our series, justifies our recommendation to rule this out with an ECG before applying the algorithm, in contrast to the AHA [1], who suggests using it only in patients who are intermediate risk. We think it is appropriate to perform a chest X-ray in patients with intermediate or low risk as it provides an alternative diagnosis in 37% of these patients. We added D-dimer measurement in patients who are low or intermediate risk, based on the results of a recent metaanalysis [18] that showed that adding this to ADD-RS reduced the error rate in these groups.
In the validation study of ADD-RS applied to the 2011 IRAD database [19], the results by risk group were similar to ours. However, in our study, all AASs were included in the categories of intermediate and high risk, with 78.9% in the high-risk group vs 59% in the 2011 study.
The main risk factor for AAS in our series was high-risk pain, the same as in the IRAD database published in 2018 [20]. We also found a similar prevalence of hypotension and hypoperfusion. We obtained discordant results for history of AA and aortic regurgitation murmur. We also found discrepancies compared with the ADD-RS validation study [19] for known aortic valve disease and recent aortic manipulation.
Recently, alternative scoring systems have been published, such as the 2020 Canadian guidelines [21], which are based on those from the AHA. In line with our results, they give greater importance to the presence of typical pain, history of AA, and new aortic regurgitation murmur. Another of these is the AORTA score [22], in which the percentage of AAS was similar to that in our study, and, as in our study, history of AA was associated with a significantly higher probability of AAS. By way of differences, both algorithms gave greater value to hypotension or shock, while in our series this variable was associated with alternative diagnoses, but not with AAS.
Our series confirms the great variability in AAS presentation, as well as conditions that may mimic AAS. We found differences compared with the 2013 study by Lovy et al. [16], with a higher prevalence of PTE and other pulmonary pathologies. Compared with the Canadian guidelines [21], we found a higher prevalence of cardiac abnormalities, PTE, and other pulmonary pathologies, which may mean that their modified algorithm was more specific for differentiating AAS from cardiac and pulmonary disease. In contrast, we had a lower prevalence of musculoskeletal pathology.
The implementation of this CDSS in our hospital notably increased detection of potentially serious pathologies that mimic AAS using CTA aorta, especially those that cause hemodynamic instability. The great variability in presentation of AAS makes it necessary to include multiple variables to increase the sensitivity of the algorithm and avoid underdiagnosis without having to perform unnecessary investigations in patients with low pretest probability.
Limitations
This CDSS was implemented in a tertiary hospital, so we cannot extrapolate the results to other non-tertiary hospitals. We did not assess the satisfaction of the professionals using the algorithm or the potential difficulties of putting the system into practice. We do not know how many AAS were not diagnosed due to incorrect application of the algorithm or incorrect stratification, so we cannot calculate the sensitivity and specificity of our score. The differences from other studies based on IRAD must be interpreted with caution, as our study, unlike IRAD, included the whole AAS spectrum.
The study period was very short for such an uncommon condition, which led to the low number of patients with a positive diagnosis of AAS. We were unable to prolong the study period due to issues with the electronic medical record system at our hospital. The number of observations and their retrospective nature are insufficient to develop a clinical prediction rule. A prospective validation study of the score with more patients is needed.
Conclusions
The use at our hospital of this evidence-based pathway for requesting CTA in patients with suspected AAS achieved an improvement in AAS management and in the diagnostic yield of this test.
All the patients who had AAS had been stratified as high or intermediate risk. The data analysis showed a higher probability of AAS, which reached statistical significance, in patients with known AA or with a murmur suggestive of aortic regurgitation. In addition, it demonstrated an increased probability of having an alternative acute serious pathology in patients with hypotension or shock.
Plain chest X-ray along with D-dimer measurement in patients with low or intermediate probability of AAS allows clinicians to determine alternative diagnoses and reduce unnecessary CTA requests.
The use of a sensitive algorithm in the emergency department that includes information on presentation, examination findings, past medical history, and other investigations can be useful to optimize the diagnosis of AAS.
Funding
This study was supported by the Instituto de Salud Carlos III (Plan Estatal de I+D+i 2013-2016) projects (P13/00896, P13/01183, P16/00296, PI16/01786, P16/01828, P16/00558) and cofinanced by the European Development Regional Fund “A way to achieve Europe” (EDRF).
References
- Writing Group Members, Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010; 121.
- Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J. 2018; 39: 739-749d.
- Salmasi MY, Al-Saadi N, Hartley P, Jarral OA, Raja S, Hussein M, et al. The risk of misdiagnosis in acute thoracic aortic dissection: a review of current guidelines. Heart. 2020; 106: 885-891.
- 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35: 2873-2926.
- Jill E Jacobs, Larry A Latson Jr., Suhny Abbara, Scott R Akers, Philip A Araoz, Kristopher W Cummings, et al. Acute Chest Pain - Suspected Aortic Dissection. 2020.
- Gunn AJ, Kalva PS, Majdalany BS, Craft J, Eldrup-Jorgensen J, Ferencik M, et al. Nontraumatic Aortic Disease. 2020.
- Vardhanabhuti V, Nicol E, Morgan-Hughes G, Roobottom CA, Roditi G, Hamilton MCK, et al. Recommendations for accurate CT diagnosis of suspected acute aortic syndrome (AAS)-on behalf of the British Society of Cardiovascular Imaging (BSCI)/British Society of Cardiovascular CT (BSCCT). Br J Radiol. 2016; 89: 20150705.
- Murayama T, Funabashi N, Uehara M, Takaoka H, Komuro I. New classification of aortic dissection during the cardiac cycle as pulsating type and static type evaluated by electrocardiogram-gated multislice CT. Int J Cardiol. 2010; 142: 177-186.
- Woo YJ, Greene CL. Clinical manifestations and diagnosis of thoracic aortic aneurysm. 2020.
- Black JH, Manning WJ. Clinical features and diagnosis of acute aortic dissection. 2020.
- Chung J. Epidemiology, risk factors, pathogenesis, and natural history of abdominal aortic aneurysm.
- Black JH, Manning WJ. Management of acute aortic dissection - UpToDate.
- Dawn Abbott J. Thoracic Aortic Aneurysm.
- Dawn Abbott J, Osborn EA. Thoracic Aortic Dissection.
- Asha SE, Miers JW. A Systematic Review and Meta-analysis of D-dimer as a Rule-out Test for Suspected Acute Aortic Dissection. Ann Emerg Med. 2015; 66: 368-378.
- Lovy AJ, Bellin E, Levsky JM, Esses D, Haramati LB. Preliminary development of a clinical decision rule for acute aortic syndromes. Am J Emerg Med. 2013; 31: 1546-1550.
- von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical Prediction of Acute Aortic Dissection. Arch Intern Med. 2000; 160: 2977.
- Tsutsumi Y, Tsujimoto Y, Takahashi S, Tsuchiya A, Fukuma S, Yamamoto Y, et al. Accuracy of aortic dissection detection risk score alone or with D-dimer: A systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2020; 9: S32-39.
- Rogers AM, Hermann LK, Booher AM, Nienaber CA, Williams DM, Kazerooni EA, et al. Sensitivity of the Aortic Dissection Detection Risk Score, a Novel Guideline-Based Tool for Identification of Acute Aortic Dissection at Initial Presentation: Results From the International Registry of Acute Aortic Dissection. Circulation. 2011; 123: 2213-2218.
- Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation. 2018; 137: 1846-1860.
- Ohle R, Yan JW, Yadav K, Cournoyer A, Savage DW, Jetty P, et al. Diagnosing acute aortic syndrome: a Canadian clinical practice guideline. Can Med Assoc J. 2020; 192: E832-843.
- Morello F, Bima P, Pivetta E, Santoro M, Catini E, Casanova B, et al. Development and Validation of a Simplified Probability Assessment Score Integrated With Age-Adjusted D Dimer for Diagnosis of Acute Aortic Syndromes. J Am Heart Assoc. 2021; 10.
Citation: Lumbreras-Fernández B, Vicente Bártulos A, Fernandez-Felix BM, Corres González J, Zamora J and Muriel A. Development of a Computerized, Evidence-Based Decision Support System for Computed Tomography Assessment in Acute Aortic Syndrome in the Emergency Department. Austin J Radiol. 2021; 8(10): 1164.