
Case Report
Austin J Radiol. 2021; 8(10): 1165.
Cystic Meningioma in an Adult: Unusual Radiologic Appearance of a Common Intracranial Tumor
Soukaina A*, Sanae S, Meryem F, Mohammed J and Firdaous T
Radiology Department in Specialities Hospital, Ibn Sina, Rabat, Morocco
*Corresponding author: Allioui Soukaina, Radiology Resident, Radiology, Department in Specialities Hospital, Ibn Sina, Rabat, Morocco
Received: September 11, 2021; Accepted: October 06, 2021; Published: October 13, 2021
Abstract
Meningiomas are common intracranial brain tumors. However, cystic meningiomas are very rare, accounting for 2%-4% of intracranial meningiomas. In this article, we report a 52 years old female patient with a parasagittal right frontal cystic meningioma type V recognized by its neuroimaging features on CT scan and MRI. Imaging modalities can play a crucial role in the appreciation of subtypes of cystic meningiomas, and the radiological analysis for appropriate surgical treatment.
Keywords: Cystic; Meningioma; CT; MRI
Introduction
Meningiomas are common extra-axial intracranial tumors that represent approximately 15% of all intracranial neoplasms. They are typically benign, although malignant forms also exist [1]. Habitually, they are highly cellular and well vascularised solid masses. However, cystic meningiomas represent a rare imaging feature and must be differentiated from other brain tumors.
Case Presentation
A 52-year-old female, with history of headaches and behaviour disturbance, was emergently referred for sudden onset of seizures and loss of conscience. The patient underwent a Computed Tomography (CT) of the head without intravenous contrast which objectified a hypodense mass in the right frontal region, with a cystic component, perifocal oedema and associated mass effect with subfalcine herniation (Figure 1). Cranial Magnetic Resonance Imaging (MRI) without and with contrast was subsequently performed, revealing an extraaxial, falco-sinusal based lesion in the right frontal lobe, measuring 56x58x62 mm. This lesion had a solid part isointense on T1, T2 and FLAIR, with intense enhancement after Gadolinium, a multilocular cystic component hypointense on T1-weighted, and hyperintense on T2-weighted images, with well-circumscribed thin wall enhanced after Gadolinium and a peripheral unilocular simple thin-walled cystic part, located between the meningioma and brain. On MR spectroscopy the solid part demonstrated an elevated choline peak and a decreased N-acetylaspartate peak, with no diffusion restriction on DWI. There was no evidence of calcification or hemosiderin on SWI. This mass was surrounded by perifocal vasogenic oedema. It was associated to thickening and enhancement of the adjacent dura and subfalcine herniation (Figure 2). Those findings were compatible with cystic meningioma type V. The mass was surgically resected. The analysis of pathologic specimen confirmed the diagnosis of cystic meningioma (WHO) grade I.
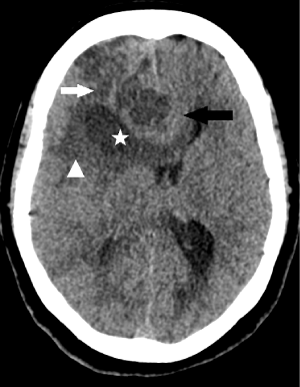
Figure 1: Axial Computed Tomography (CT) image of the head without
intravenous contrast show hypodense mass in the right frontal region (white
arrow), with a cystic component (asterisk), perifocal oedema (head of arrow)
and associated mass effect with subfalcine herniation (black arrow).
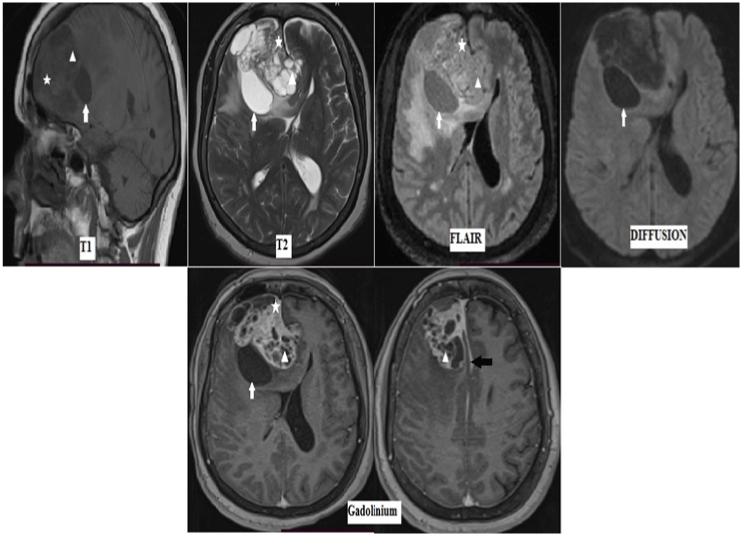
Figure 2: MRI brain showing an extra-axial mass, falco-sinusal based, in the right frontal region. The lesion is mixte, demonstrating a solid part isointense on T1, T2
and FLAIR, with intense enhancement after Gadolinium (asterisk). A multilocular cystic component hypointense on T1-weighted, and hyperintense on T2-weighted
images (head of arrow), with well-circumscribed thin wall enhanced after Gadolinium and a peripheral unilocular simple thin-walled cystic part, located between the
meningioma and brain (white arrow). This mass does not show diffusion restriction on DWI. It is associated to thickening and enhancement of the adjacent dura
(black arrow).
Discussion
Meningiomas are benign tumors which originate from the meningothelial cells; they are the most common intracranial tumor [2]. Cystic meningiomas are frequent in infants and children accounting for approximately 10-19% of meningiomas, but they are scarce in adults (2-4%) [3]. Their aetiology is obscure. However, some elements comprising traumatic brain injury, type II neurofibromatosis and radiation exposure are known as predisposing factors to these tumors [4].
Two classification systems are useable for cystic meningioma, presented by Nauta et al. in 1979 and Rengachary et al. Based on the latter, cystic meningiomas may be grouped as intratumoral and peritumoral cystic meningiomas. According to the relationship between the tumoral mass and the cystic cavity, Nauta et al patterned cystic meningiomas into four types, as follows: I-with intratumoral cyst(s), situated centrally within the meningioma; II- with intratumoral cyst(s), located peripherally within the meningioma but still surrounded by tumor; III- cyst wall may contain nest of tumor cells; IV- with cyst(s) located within the adjacent brain. Worthington appended an additional type V, where cysts are situated between the meningioma and brain. Our patient had Type V cystic meningioma [5-7].
Some theories on the pathogenesis of cystic lesions are described in the literature. Types I and II could be due to microcystic degeneration, ischemic necrosis, intratumoral haemorrhage or active secretion from tumor cells. Type III can be the result of reactive gliosis or cerebral oedema, type IV may be form as a result of widened subarachnoid spaces and trapped cerebrospinal fluid around the tumor [8].
Brain MRI is the modality of choice for diagnosis of cystic meningioma, with higher precision than CT [9]. Cystic meningiomas are extra-axial masses, well circumscribed, with intense and homogeneous enhancement of the solid part after contrast on both CT and MRI. The cystic component may demonstrate wall enhancement. The absence of this latter does not eliminate the presence of tumor cells [10]. Meningiomas may be associated to thickening of the adjacent dura (dural tail sign) which could be present in some tumors such as schwannomas, glioblastomas and metatsases, and surrounded by perifocal oedema which may suggest glioma [11]. Meningiomas have variable comportment according to the degree of cellularity in diffusion weighted images (DWI), which make the relationship between Apparent Diffusion Coefficient (ADC) and histopathology not clear. In our case, the mass showed no restriction [12].
Spectroscopy shows elevated choline peak, decreased N-acetylaspartate peak and NAA/Cho ratio. Additionally, increased lipid and alanine peaks could be found at 1.5ppm, which may distinguish meningiomas from other neoplasms [13].
Treatment choices are based on multiples factors, comprising age of the patient, associated comorbidities, location of the tumor and histological subtype. Surgery is indicated if the lesion is symptomatic. Asymptomatic patients with small meningiomas can be followed with serial imaging studies. In cases of malignant meningiomas, radiation therapy can also be used [14].
Conclusion
Cystic meningiomas are rare tumors. A rigorous diagnosis may be based on CT and MRI with spectroscopy. The division of cysts into five groups may help the neurosurgeon to realise total resection, and limit the risk of recurrence of these tumors.
References
- Amit Mittal, Kennith F. Layton, S. Sam Finn, George J. Snipes and Michael J. Opatowsky. Cystic meningioma: unusual imaging appearance of a common intracranial tumor. Proc (Bayl Univ Med Cent). 2010; 23: 429-431.
- MP Buetow, PC Buetow and JG Smirniotopoulos. “Typical, atypical, and misleading features inmeningioma”. Radio Graphics. 1991; 11: 1087-1106.
- Kejriwal GS, Madhavi C, Sahu SN. Cystic Meningioma. Bangladesh Journal of Medical Science. 2013; 12: 83-85.
- Aikaterini Solomou, Pantelis Kraniotis. Cystic meningioma in an adult. Euroradio. 2016.
- HJW Nauta, WS Tucker, WJ Horsey, JM Bilbao and C Gonsalves. “Xanthochromic cysts associated with meningioma.” Journal of Neurology Neurosurgery and Psychiatry. 1979; 42: 529-535.
- M Rinaldi, E Mezzano, M Berra, R Olocco, H Pares and F Papalini. “Variante poco frecuente de meningiomas: meningiomas qu´isticos”. Revista Argentina de Neurocirug. 2008; 22: 107-109.
- Worthington C, Caron JL, Melanson D and Leblanc R. Meningioma cysts. Neurology. 1985; 35: 1720-1724.
- Fortuna A, Ferrante L, Acqui M, Guglielmi G, Mastronardi L. Cystic meningiomas. Acta Neurochir. 1988; 90: 23-30.
- Ferrante L, Acqui M, Lunardi P, Qasho R and Fortuna A. MRI in the diagnosis of cystic meningiomas: Surgical implications. Acta Neurochir (Wien). 1977; 139: 8-11.
- Arai M, Kashihara K and Kaizaki Y. Enhancing gliotic cyst wall with microvascular proliferation adjacent to a meningioma. J Clin Neurosci. 2006; 13: 136-139.
- CS Zee, T Chen, DR Hinton, et al. “Magnetic resonance imaging of cystic meningiomas and its surgical implications”. Neurosurgery. 1995; 36: 482-488.
- Tai-Youeng Chen, Ping-Hong Lai, Jih-Tsun Ho, Jyh-Seng Wang, Wei-Liang Chen, Huay-Ben Pan, et al. Magnetic resonance imaging and diffusionweighted images of cystic meningioma Correlating with histopathology. Journal of Clinical Imaging. 2004; 28: 10-19.
- N Bulakbasi, M Kocaoglu, F ¨Ors, C Tayfun and T ¨ Ug¨oz. “Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors”. American Journal of Neuroradiology. 2003; 23: 225-233.
- Falavigna A, Santos JA, Chimelli L, Ferraz FA, Bonatelli Ad Ade P. Anaplastic meningioma: case report. Arq Neuropsiquiatr. 2001; 59: 939-943.