Review Article
Austin J Reprod Med Infertil. 2014;1(1): 5.
Fine Structural Markers of Human Oocyte Quality in Assisted Reproduction
Nottola SA1*, Macchiarelli G2 and Familiari G1
1Department of Anatomy, Histology, Forensic Medicine and Orthopaedics, Sapienza University, Italy
2Department of Life, Health and Environmental Sciences, University of L´Aquila, Italy
*Corresponding author: Nottola SA, Department of Anatomy, Histology, Forensic Medicine and Orthopaedics, Section of Human Anatomy, Electron Microscopy Unit, Laboratory “Pietro M. Motta”, Sapienza University of Rome, Via Alfonso Borelli 50, Rome 00161, Italy
Received: September 30, 2014; Accepted: November 27, 2014; Published: December 02, 2014
Abstract
After completing its maturation, the human oocyte acquires very peculiar fine structural features. In this review we describe by LM, TEM and SEM the nuclear and cytoplasmic changes, as well as the changes in the texture of the ZP, occurring in the human oocyte during the final stages of maturation. Oocytes were obtained during ART cycles and destined to morphological evaluation after the informed consent of the patients. The critical analysis of the data reviewed leads us to emphasize that: 1. Both the completion of the maturative changes in the preovulatory period and the absence of degenerative alterations in the cytoplasmic microdomains of the human mature oocyte ultimately render the female gamete competent for fertilization; 2. Several minute cellular aberrations, detectable only by electron microscopy analysis, may occur in the human oocyte as the consequence of the application of ART protocols (IVM and cryopreservation, in particular) and could be responsible for ART failures, even affecting early embryo development.
Keywords: Oocyte; Assisted reproduction; In vitro maturation; Cryopreservation; Human; Electron microscopy
Abbreviations
ART: Assisted Reproductive Technology; GV: Germinal Vesicle; GVBD: Germinal Vesicle Breakdown; IVM: In vitro Maturation; LM: Light Microscopy; M-SER: Mitochondria-smooth Endoplasmic Reticulum; MII: Second Metaphase; MV: Mitochondria-vesicle; PVS: Perivitelline Space; SEM: Scanning Electron Microscopy; SER: Smooth Endoplasmic Reticulum; TEM: Transmission Electron Microscopy; ZP: Zona Pellucida
Introduction
The positive outcome of ART procedures strictly depends on the oocyte quality, evaluable in terms of maintenance of full morphofunctional integrity during its complex and long maturative iter. In fact, in vivo and in vitro as well, the completion of oocyte maturative changes in the preovulatory period and the absence of degenerative alterations in the oocyte cytoplasmic microdomains both ultimately render the female gamete competent for fertilization.
Aim of this paper is to briefly review the process of the periovulatory maturation of the oocyte in humans, giving particular emphasis to its fine structural morphology, as revealed by LM, TEM and SEM [1- 7]. Electron microscopy observations in particular, associated with clinical, epidemiological, biological, molecular and biochemical data, are useful in the assessment of the structural integrity of the oocyte, in order to define a proper “oocyte health” state. Electron microscopy, in fact, can highlight not only obvious defects but even minute subcellular alterations in the oocyte microdomains. These minimal alterations cannot be diagnosed by other morphological approaches routinely used in ART laboratories and could be responsible, in some cases, of unexplained failures of ART procedures [1]. Recently, electron microscopy, applied to the study of human oocytes subjected to different protocols aimed at the preservation of female fertility, has also provided some useful and objective information regarding oocyte sensitivity to cryopreservation [8-17] and/or to IVM [15].
Methodology
The data reported in this review mostly derived from structural and ultrastructural observations carried out by our research groups on human oocytes obtained during ART cycles and evaluated at the light of the specific literature at regard. All the samples, destined to electron microscopy observations after the informed consent of the patients, were fixed in 0.5% - 2% glutaraldehyde in phosphate buffer and processed for LM, TEM and SEM as detailed elsewhere [6, 8-10, 13,18,19].
The Human Mature Oocyte: Fine Structural Parameters
General features
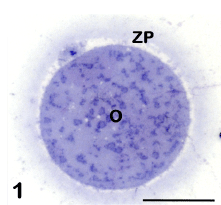
Observed by LM in equatorial sections, the human mature oocyte shows a rounded shape and a diameter of about 100 μm. The ooplasm has a homogeneous texture and contains numerous, uniformly dispersed organelles. Vacuoles, variable in size and location, are only occasionally observed in healthy oocytes. The oocyte is surrounded by the ZP, a glycoprotein matrix that forms a continuous and uniform layer and has a thickness of about 17 μm. The oocyte and ZP are separated by a thin PVS, in which the first polar body is located, visible only in particularly favorable sections (Figure 1). In the same sections the MII chromosomes are also frequently observed, aligned on the meiotic spindle [8-10,13]. Further details on each of these structures will be provided in the next paragraphs.
Figure 1: Mature oocyte (O) surrounded by the zona pellucida (ZP). The first polar body is visible in the perivitelline space. LM, bar is 45 μm.
Chromosomes and meiotic spindle of the second metaphase
In order to become fertilizable, the oocyte destined to ovulation undergoes a series of maturative changes that affect both nuclear and cytoplasmic domains.
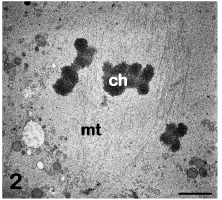
As far as the nuclear changes are concerned, the first response of the oocyte to the ovulatory surge of luteinizing hormone and to the removal of inhibitory intrafollicular influences is the resumption of meiosis [7]. A series of ultrastructural features characterize this process. Before resuming meiosis, a voluminous nucleus, called GV, is present in the oocyte, arrested in the prophase of the first meiotic division. The GV, observed by TEM, appears as a rounded structure, which usually assumes an eccentric position in the ooplasm. The nuclear envelope (nucleolemma) is slightly undulated and provided with numerous pores. The nucleoplasm contains one or more evident nucleoli, with a compact texture, associated with clumps of dense chromatin. The remaining chromatin is finely dispersed in the nucleoplasm. When oocyte resumes meiosis the GVBD takes place. In the initial stages of the GVBD chromatin condenses to form chromosomes and the nucleolus disappears. The nuclear envelope becomes highly folded and ultimately breaks, leading to the release of the chromosomes at the periphery of the oocyte. The first meiotic spindle forms and the first meiotic division rapidly occurs, culminating with the extrusion of the first polar body in the PVS. Afterwards, the second meiotic division immediately starts and stops again at the MII stage, to be completed only at fertilization, with the extrusion of the second polar body [3]. Thus in the mature, fertilizable oocyte, MII chromosomes can be seen by TEM arranged onto the equatorial plate of the meiotic spindle. The chromosomes have a strongly electron dense, granulo-fibrillar microstructure (Figure 2). The meiotic spindle is located in a peripheral region of the ooplasm, usually devoid of other organelles, and, in humans, it shows an orientation perpendicular to the oolemma. The spindle is anastral, devoid of centrioles and has a barrel shape, with slightly sharp poles. It is composed of numerous microtubules which extend from one pole to the other (Figure 2) [4].
Figure 2: Mature oocyte. The metaphase II spindle is composed of microtubules (mt) and associated chromosomes (ch). TEM, bar is 1,5 μm. [9].
Ooplasm
In general, oocyte organelles become far more abundant during final oocyte growth. The main fine structural cytoplasmic markers of maturity in the human oocyte are: 1. the occurrence of numerous cytoplasmic aggregates, mainly formed by mitochondria and cytoplasmic membranes, and 2. the distribution of CGs just beneath the oolemma [1,20-22].
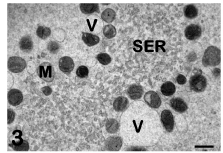
Cytoplasmic aggregates: Mitochondria are the most abundant organelles in the ooplasm of the human mature oocyte. They are spherical or oval in shape, and contain a dense matrix and a few peripheral, arched or transversal, cristae [2,23]. Some mitochondria may present clear vacuoles within their matrix. In the human mature oocyte, mitochondria are characteristically associated with SER membranes, resulting in the formation of peculiar structures mainly located in the cortical ooplasm [3,4]. These structures are composed of voluminous aggregates of anastomosing tubules or appear as small vesicles containing a flocculent, slightly electron dense material. Both tubules and vesicles appear surrounded by mitochondria and are named M-SER aggregates and MV complexes, respectively (Figure 3). Although SER elements may dynamically acquire different shapes (tubules or vesicles) and appear as independent structures by TEM, actually they belong to the same system of interconnected membranes. In fact, transitional figures with intermediate characteristics between tubules and vesicles have been observed by TEM [1]. In this regard, during the maturation of the oocyte, the aggregation of SER tubules appears to be the first phenomenon to occur. Subsequently, these tubules become surrounded by mitochondria, forming mitochondria- SER aggregates. Small MV complexes, found in both immature (GV-stage) and mature oocytes [5,17], may also provide membranes and mitochondria for the assembly of mitochondria-SER aggregates.
Figure 3: Mature oocyte. Tubules (SER) and vesicles (V) of smooth endoplasmic reticulum are associated with mitochondria (M) to form M-SER aggregates and MV complexes. TEM, bar is 0,5 μm. [10].
Different roles are hypothesized for these peculiar membrane systems and associated mitochondria. They may co-work to obtain: a. Rich pool of energy reserve; b. Production/secretion of substances (nutrients, growth factors) useful for fertilization; c. Rapid neogenesis of plasma and nuclear membranes during the first embryo cleavages [1,2,23]. Aggregates between mitochondria and endoplasmic reticulum membranes also appear to be involved in the mechanisms of storage and release of Ca++, which plays a key role at the time of fertilization [7,24].
The presence of very large mitochondria-SER aggregates (so large to be in some cases visible not only by TEM but also by phase contrast microscopy examination during ART procedures) may occur as the effect of gonadotropin stimulation [3] and has been put in relation with an altered embryonic and fetal development [24,25], although different opinions have been recently expressed [26].
TEM also allows the detection of numerous, large, atypical MV complexes in human oocytes that have been subjected to IVM or are kept in culture for a long time. These complexes, presumably deriving from a further remodeling of the above described cytoplasmic aggregates, can be identified as fine structural markers of prolonged culture [3-5,15,16].
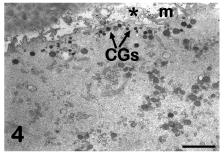
Cortical granules: CGs originates from the Golgi apparatus in the immature, GV oocytes and progressively increases in number during oocyte maturation, migrating toward the oolemma. In human mature oocytes, CGs is stratified in two/three rows in subplasmalemmal areas. By TEM, CGs appears as membrane-bounded organelles with a diameter of about 0.2-0.6 μm and a very dense, homogeneous matrix (Figure 4). CGs contains mucopolysaccharides, proteases, tissue type plasminogen activator, acid phosphatase and peroxidase. Although some of these granules may occasionally discharge their content independently of fertilization, nevertheless a sudden and massive physiological release occurs only at fertilization time. The release of CG content in the PVS at fertilization (“cortical reaction”) and the consequent compaction and hardening of the inner aspect of the ZP (“zona reaction”) are finalized to prevent the penetration of supernumerary spermatozoa into the oocyte (polyspermy) [4,6,27].
Figure 4: Mature oocyte. A rim of cortical granules (CGs) is seen under the oolemma. M: microvilli, asterisk: perivitelline space. TEM, bar is 2 μm. [9].
Isolated CGs, still migrating toward the cell periphery, has been sometimes detected in the inner ooplasm of IVM oocytes, confirming the possible occurrence of an incomplete cytoplasmic maturation in these oocytes [15]. In addition, CGc appear generally sparse, forming a discontinuous layer in cryopreserved oocytes, irrespective of the protocol applied (slow freezing or vitrification). This latter finding suggests that a precocious oocyte activation may be triggered by cryopreservation, leading to a premature CG exocytosis and ZP hardening [8-10,12-17,28]. Both ART procedures (IVM and cryopreservation) may thus impair oocyte competence to fertilization, determining a defective distribution of CGs.
Other organelles: Contrarily to what previously reported for the SER, membranes of rough endoplasmic reticulum are mostly absent in the ooplasm of the human mature oocyte, and also free ribosomeís are rarely encountered. Even the Golgi complex, after having exhausted its role in producing CGs, is only occasionally observed in the mature oocyte, becoming gradually replaced by SER during the final stages of oocyte maturation. All these fine structural features indicate a relative inactivity of human mature oocytes in protein synthesis and secretion [3].
Differently to what occurs in other mammalian species (cattle and sheep) vacuoles are almost completely absent in the human mature oocyte. When found, vacuoles can be identified by TEM as rounded, apparently empty structures surrounded by a membrane often interrupted (Figure 5). The presence of multiple vacuoles of various sizes is considered a sign of early degeneration of the oocyte [8,9] and may affect the future development of the embryo [29]. In fact, although oocytes with vacuoles can be fertilized, they rarely divide [5]. Lysosomes and multivesicular bodies, organelles both correlated to the triggering of phenomena of autolysis/autodigestion, often associate with vacuoles in the ooplasm of damaged oocytes (Figure 5) [4].
Figure 5: Early degenerating mature oocyte. Numerous vacuoles (Va) associated with lysosomes (Ly) are found in the ooplasm. TEM, bar is 1,2 μm. [9].
During the application of cryopreservation protocols, a slight to moderate vacuolization has been observed in the ooplasm of human oocytes subjected to slow freezing [8,9,13,16], whereas vacuoles appear generally scarce when oocytes are vitrified, particularly if open cryodevices, such as cryoloop or cryoleaf, are employed [10]. Thus, on the basis of these data, vacuole formation after cryopreservation may be judged one of the most accurate ooplasmic markers of oocyte sensitivity to cryodamage. The presence and extent of vacuolization may be also depending, at least in part, on the protocol of cryopreservation applied.
Oolemma and perivitelline space
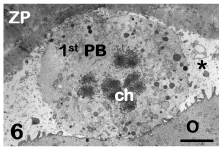
The human mature oocyte possesses very numerous microvilli projecting into the PVS (Figure 4). These microvilli exert a decisive role at fertilization, mediating the initial contact and fusion of the fertilizing sperm with the oolemma [30]. The PVS, that is narrow in healthy oocytes, contains the first polar body in which chromosomes, associated to residual microtubules, and CGs can be seen by TEM (Figure 6) [3-5].
Figure 6: Mature oocyte. The first polar body (1st PB) with its chromosomes (ch) is visible in the perivitelline space (asterisk). O: oocyte, ZP: zona pellucida. TEM, bar is 2,5 μm. [9].
The presence of an enlarged PVS, especially if associated with obvious alterations of the morphology of the oocyte, such as: increased density of the cytoplasmic matrix, presence of an extensive vacuolization, and reduction in amount of organelles and/or of microvilli, indicate a frank atresia of the oocyte [1].
Zona pellucida
The ZP is an amorphous extracellular matrix that is formed during the development of the preantral follicle at the interface between oocyte and follicular cells. Once produced, the ZP remains around the oocyte during the later stages of folliculogenesis, at the time of ovulation and fertilization, and thereafter continues to surround the embryo during its first divisions until implantation. The ZP, acting as a protective and selective barrier, actually mediates the metabolic exchanges between the oocyte/embryo and the surrounding microenvironment [2].
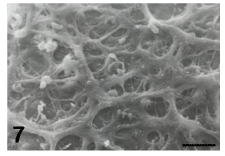
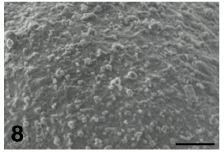
By TEM, the ZP appears composed of a very fine texture of filaments and granular agglomerates [31]. By SEM, which provides a vivid, three-dimensional surface view of cells and tissues, the outer aspect of the ZP of mature and healthy oocytes shows the presence of numerous fenestrations (Figure 7). These latter consist of a loose network of microfilaments, which give to the ZP a characteristic spongy appearance. Immature or atretic oocytes are instead surrounded by a ZP whose outer surface shows a smooth, compact texture (Figure 8), with filaments arranged to form a very dense network. At fertilization, spermatozoa can be actually “captured” by the meshes of the spongy zona, where specific binding sites for the spermatozoa are presumably located. On the contrary, the condensation of the outer portion of the ZP covering immature or atretic oocytes probably causes a “disorientation” of the same sites, preventing sperm binding and penetration and ultimately impairing oocyte fertilizability [31-33]. The inner surface of the ZP of mature oocytes, when observed by TEM and SEM, appears formed by thin filaments anastomosed to each other and interconnected by small globular structures. After fertilization, while the outer portion of the ZP maintains a spongy appearance [19], the inner side shows a diffuse condensation of the filaments, interpretable as a fine structural marker of the occurrence of the zona reaction [31].
Figure 7: Mature oocyte. Note the spongy appearance of the outer aspect of the zona pellucida. SEM, bar is 3 μm.
Figure 8: Immature/atretic oocyte. Note the compact texture, without fenestrations, of the outer aspect of the zona pellucida. SEM, bar is 7 μ.
Conclusions and Future Perspectives
In conclusion, during the final stages of maturation, the human oocyte undergoes a complex, ordered and coordinated reorganization of its genome, ooplasm and surrounding extracellular matrix. This reorganization, followed by electron microscopy, appears to be characterized by neogenesis, modification and redistribution of organelles, membranes and glycoproteinís in the ZP. A spatial and/ or temporal alteration of these processes, which may occur in vivo and during the application of ART protocols as well, can be without doubts responsible for a reduced or impaired performance of the oocyte at fertilization. In addition, the efficacy of the above processes is not only essential for rendering the oocyte fertilizable, but is also crucial for the healthy development of the future embryo. At this regard, it is also worth noting that a peculiar cytoplasmic inheritance in the zygote is mostly derived from molecules and organelles properly stored in the oocyte, mainly mitochondria that with their own DNA (mitochondrial DNA) are transmitted from one generation to the next through the oocyte cytoplasm [23,34].
The acquisition of oocyte competence to be fertilized and generate a healthy embryo can be also further improved by the presence, in vitro, of other components of the ovarian follicle, such the cumulus-corona cells [18,35] and even the parietal granulosa cells used for co-cultures [36]. All these somatic cells of the follicle, in fact, possess the morphofunctional correlates of actively secreting cells capable of creating around the oocyte/early embryo a suitable microenvironment enriched of “embryotrophic” factors. In our opinion, this somatic support deserves to be more deeply investigated in different ART conditions.
Finally, the definition and standardization of fine structural markers of quality for the human mature oocyte could be also useful for a constructive evaluation of the morphology of the oocytes subjected to different ART procedures, cryopreservation in particular. The final goal will be to give a contribution to the identification of less aggressive protocols in terms of preservation of oocyte quality and viability in ART.
References
- Motta PM, Nottola SA, Micara G, Familiari G. Ultrastructure of human unfertilized oocytes and polyspermic embryos in an IVF-ET program. Ann N Y Acad Sci. 1988; 541: 367-383.
- Motta PM, Nottola SA, Familiari G, Makabe S, Stallone T, Macchiarelli G. Morphodynamics of the follicular-luteal complex during early ovarian development and reproductive life. Int Rev Cytol. 2003; 223: 177-288.
- Sathananthan AH, Ng S-C, Bongso A, Trounson A, Ratnam SS. Visual Atlas of Early Human Development for Assisted Reproductive Technology. Singapore: National University of Singapore. 1993.
- Sathananthan AH, Selvaraj K, Girijashankar ML, Ganesh V, Selvaraj P, Trounson AO. From oogonia to mature oocytes: inactivation of the maternal centrosome in humans. Microsc Res Tech. 2006; 69: 396-407.
- El.Shafie M, Sousa M, Windt M-L, Kruger TF. An Atlas of the Ultrastructure of Human oocytes. New York: Parthenon Publishing. 2000.
- Familiari G, Heyn R, Relucenti M, Nottola SA, Sathananthan AH. Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int Rev Cytol. 2006; 249: 53-141.
- Makabe S, Van Blerkom J, Nottola SA, Naguro T. Atlas of Human Female Reproductive Function. Ovarian Development to Early Embryogenesis after In-vitro Fertilization. London: Taylor and Francis. 2006.
- Nottola SA, Macchiarelli G, Coticchio G, Bianchi S, Cecconi S, De Santis L, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation using different sucrose concentrations. Hum Reprod. 2007; 22:1123-1133.
- Nottola SA, Coticchio G, De Santis L, Macchiarelli G, Maione M, Bianchi S. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod Biomed Online. 2008; 17: 368-377.
- Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online. 2009; 19 Suppl 3: 17-27.
- Camboni A, Martinez-Madrid B, Dolmans MM, Amorim CA, Nottola SA, Donnez J, et al. Preservation of fertility in young cancer patients: contribution of transmission electron microscopy. Reprod BioMed Online 2008; 17: 136-150.
- Bonetti A, Cervi M, Tomei F, Marchini M, Ortolani F, Manno M. Ultrastructural evaluation of human metaphase II oocytes after vitrification: closed versus open devices. Fertil Steril. 2011; 95: 928-935.
- Coticchio G, Borini A, Distratis V, Maione M, Scaravelli G, Bianchi V, et al. Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. J Assist Reprod Genet. 2010; 27: 131-140.
- Gualtieri R, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011; 26: 2452-2460.
- Shahedi A, Hosseini A, Khalili MA, Norouzian M, Salehi M, Piriaei A, et al. The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol. 2013; 167: 69-75.
- Bianchi V, Macchiarelli G, Borini A, Lappi M, Cecconi S, Miglietta S, et al. Fine morphological assessment of quality of human mature oocytes after slow freezing or vitrification with a closed device: a comparative analysis. Reprod Biol Endocrinol. 2014; [in press].
- Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, et al. Ultrastructure of Immature and Mature Human Oocytes after Cryotop Vitrification. J Reprod Dev. 2014.
- Nottola SA, Familiari G, Micara G, Aragona C, Motta PM. The ultrastructure of human cumulus-corona cells at the time of fertilization and early embryogenesis. A scanning and transmission electron microscopic study in an in vitro fertilization program. Arch Histol Cytol. 1991; 54: 145-161.
- Nottola SA, Makabe S, Stallone T, Familiari G, Correr S, Macchiarelli G. Surface morphology of the zona pellucida surrounding human blastocysts obtained after in vitro fertilization. Arch Histol Cytol. 2005; 68: 133-141.
- Sundström P, Nilsson BO, Liedholm P, Larsson E. Ultrastructure of maturing human oocytes. Ann N Y Acad Sci. 1985; 442: 324-331.
- Sundström P, Nilsson BO, Liedholm P, Larsson E. Ultrastructural characteristics of human oocytes fixed at follicular puncture or after culture. J In Vitro Fert Embryo Transf. 1985; 2: 195-206.
- Szöllösi D, Mandelbaum J, Plachot M, Salat-Baroux J, Cohen J. Ultrastructure of the human preovulatory oocyte. J In Vitro Fert Embryo Transf. 1986; 3: 232-242.
- Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000; 15 Suppl 2: 129-147.
- Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011; 96: 143-149.
- Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004; 19: 1591-1597.
- Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013; 28: 2111-2117.
- Sathananthan AH, Ng SC, Chia CM, Law HY, Edirisinghe WR, Ratnam SS. The origin and distribution of cortical granules in human oocytes with reference to Golgi, nucleolar, and microfilament activity. Ann N Y Acad Sci. 1985; 442: 251-264.
- Khalili MA, Maione M, Palmerini MG, Bianchi S, Macchiarelli G, Nottola SA. Ultrastructure of human mature oocytes after vitrification. Eur J Histochem. 2012; 56: e38.
- Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005; 83: 1635-1640.
- Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, et al. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol. 2007; 304: 317-325.
- Familiari G, Nottola SA, Macchiarelli G, Micara G, Aragona C, Motta PM. Human zona pellucida during in-vitro fertilization: An ultrastructural study using saponin, ruthenium red, and osmium-thiocarbohydrazide. Mol Reprod Dev. 1992; 32: 51-61.
- Familiari G, Nottola SA, Micara G, Aragona C, Motta PM. Is sperm-binding capability of the zona pellucida linked to its surface structure? A scanning electron microscopic study of human in vitro fertilization. J In vitro Fert Embryo Transf. 1988; 5: 134-143.
- Familiari G, Relucenti M, Heyn R, Micara G, Correr S. Three-dimensional structure of the zona pellucida at ovulation. Microsc Res Tech. 2006; 69: 415-426.
- Jansen RP, de Boer K. The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol. 1998; 145: 81-88.
- Motta PM, Nottola SA, Pereda J, Croxatto HB, Familiari G. Ultrastructure of human cumulus oophorus: a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod. 1995; 10: 2361-2367.
- Nottola SA, Heyn R, Camboni A, Correr S, Macchiarelli G. Ultrastructural characteristics of human granulosa cells in a coculture system for in vitro fertilization. Microsc Res Tech. 2006; 69: 508-516.