
Research Article
Austin J Reprod Med Infertil. 2015;2(1): 1006.
Treatment for High Levels of Sperm DNA Fragmentation and Nuclear Decondensation: Sequential Treatment with a Potent Antioxidant Followed by Stimulation of the One-Carbon Cycle vs One-Carbon Cycle Back-up Alone
Edouard Amar¹, Dominique Cornet², Marc Cohen³ and Yves Ménézo4*
¹Cabinet Medical Andrology, France
²Clinique de la Muette, France
³Clinique Natecia, France
4Laboratoire Clément, France
*Corresponding author: Yves Ménézo, Laboratoire Clément, France
Received: February 17, 2015; Accepted: March 15, 2015; Published: April 02, 2015
Abstract
Current lifestyle can have a negative impact on spermatogenesis via fragmentation of sperm DNA. Attempting to limit generation of ROS-related DNA damage through anti-oxidant supplementation is a tempting option, but current antioxidant treatments are inefficient, and lead to degradation of tertiary structures in sperm nuclear DNA (decondensation) by opening protaminecystine bridges. We investigated the effects of two different treatment regimes in patients, with no major semen anomalies and partners with no female factor detected. All the male partner had a very high level of sperm DNA fragmentation and de condensation: sequential treatment with a potent antioxidant followed by support of the one carbon cycle was compared with one-carbon cycle support alone. These two groups were also compared with a third (control) group of men who chose not to take these supplements. All of the patients who received sequential treatment showed a decrease in their fragmentation index, without concomitant increase in nuclear decondensation. However, patients treated with one-carbon cycle support alone showed similar results. The pregnancy rates reached 50% and over in the treated groups vs 27% in the control group. This confirms a strong correlation between methylation, homocysteine recycling and oxidative stress (Hoffman 2011, Ménézo et al. 2011).
Keywords: Sperm; DNA fragmentation; Nucleus decondensation; Antioxidants; One carbon cycle
Introduction
Male reproductive failure is thought to represent more than one half of infertility cases in Western countries. Until the 1980’s, semen analysis focused only on morphology, volume motility and sperm count. The pioneering work of Evenson et al. [1] led to a better appreciation of sperm quality through estimation of DNA integrity: fragmentation (DNA fragmentation index, DFI) and sperm nuclear decondensation index (SDI), or high DNA stainability (HDS) in a sperm chromatin structure assay (SCSA®). DNA fragmentation is also measured by the Halo test (SCD) and the Comet assay. DFI and SDI do not necessarily correlate with the classical WHO sperm parameters of count, motility and morphology and without treatment are relatively stable over time [2-4]. The DFI increases considerably with age [2,4], but SDI decreases slightly, indicating an age-related weakened defense against reactive oxygen species [2-5].
In theory, such chemical insults can be repaired by the oocyte/ zygote [6-8], but this capacity is finite and decreases with age. If the oocyte is overwhelmed by oxidative insult, DNA damage is not repaired, and this may lead to mutations and subsequently to miscarriages or later pathologies [9-15].
Recent observations in animals [16] have emphasized potential risks to postnatal development after performing micro-injection with highly fragmented DNA. Oxidative stress (OS) is one of the major causes of DNA and chromatin damage, with an impact on sperm quality [17,18]. Reactive oxygen species (ROS) originate partly, but not entirely from the spermatozoon itself, originating in the mitochondria [19]. Attempting to reduce or even avoid the generation of DNA damage related to ROS through oral consumption of antioxidants is tempting, particularly if we consider that the oocyte has to manage its own burden of DNA damage [17]. Mixtures of vitamins A, E and C, often coupled with selenium and coQ10 are the favorite “cocktail”. They lead to a certain extent, to some improvement [20]. However, vitamin A and E can be either anti- or pro-oxidant. Moreover, Vitamin C opens disulfide bridges [21] leading to nuclear decondensation in the spermatozoon. DNA decondensation induces chromosomal anomalies, and thus is deleterious for early development [22-23]. We recently observed [24] a similar de condensation process after treatment with mixtures of selenium and vitamins A, C, and E. Genetic and epigenetic components both play a part in paternally transmitted DNA damage to the offspring. Moreover, oxidized glutathione is mandatory for padlocking and cross-linking protamines [25]: An equilibrium between oxidation and reduction status has to be maintained over time. In fact, although ‘blanket’ ingestion of anti-oxidants containing vitamins A, C and E is at present far merely highly questionable [26], there is no longer any question regarding the strong correlation between oxidative stress, DNA methylation and its further impact on transmitted diseases [14,27-31] and this can be easily explained in terms of biochemistry (Figure 1). Oxidative stress has an impact on the one-carbon cycle, which is involved in homocysteine recycling and formation of methionine, methylation and compaction of sperm DNA [5,32-34] Through cysteine synthesis, the one-carbon cycle also facilitates the synthesis of two major endogenous antioxidants: hypotaurine [35] and glutathione.
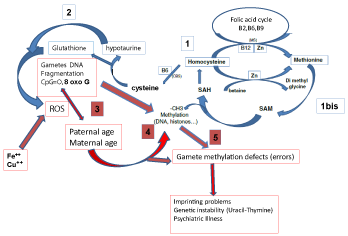
Figure 1: Interrelations between oxidative stress and methylation processes.
1- Correct recycling of Homocysteine allows generation of Cysteine and
methionine which allows correct processes of methylation (1bis).
2- Correct generation of cysteine allows synthesis of Hypotaurine and
glutathione, two potent inhibitors of ROS.
3- Generation of ROS induces DNA fragmentation. Advanced age decreases
the ability to control ROS-linked decays.
4-High levels of homocysteine inhibit methylation processes in sperm
physiology (methylation of the membrane lipids) and in sperm DNA.
5 - DNA methylation defects, whether or not linked to imprinting may result
in negative transgenerational health problems. Unrepaired 8 oxoG leads
to aberrant methylation at CpG sites, impair transcription and may affect
telomere length.
We compared sequential treatment with a potent antioxidant followed by stimulators of the one-carbon cycle vs. stimulation of the one-carbon cycle alone, in a non- randomized cohort of patient volunteers who had been shown to have high levels of sperm DNA fragmentation(DFI), +/- associated high decondensation index (SDI).
Materials and Methods
The protocol has been performed from January 2013 to August 2013, in an ART center performing 1800 IVF/ICSI attempts per year. All subjects gave their informed consent to participate. The study was performed at a French private clinic not required to have and not having a reference Institutional Review Board, however the study was conducted in full conformity with the current revision of the Declaration of Helsinki.
All the couples have been followed up to December 2013 at the time of data collection. The pregnancies were then followed. All of the female partners had no detectable pathologies: normal ovulatory profile, endocrinological parameters (FSH< 8UI/L, estradiol<40μg/L) and ovarian reserve (AMH>2μg/l). The female partners were not older than 43, as recommended by the French Biomedicine Agency (moreover the patients over 43 are not reimbursed by the Social security). This pilot study was not randomized with a placebo group: the control group consisted entirely of male patients who received no complementary treatment during their ART cycles. All of the male partners were under consultation for primary infertility (duration >3 yrs) with previous IVF/ICSI attempts, and no pregnancies with a previous partner. All had been tested for testicular volume and varicocele by testicular palpation and sonography, and testosterone levels were in the normal range. Oligoasthenospermic patients were excluded. The total sperm count and viability (eosin-nigrosine on at least 200 cells) in the ejaculates were performed according to WHO (2010) guidelines in an accredited laboratory (ISO 15189). All total counts in the ejaculates were above the WHO threshold for infertility, and motility was >15%. Sperm DFI was measured with the flow cytometry terminal deoxynucleotidyl transferase dUTP nick end labeling (Tunel) assay [36]. The SDI was measured by microscopy of 200 cells using aniline blue (AB) [37] since it has been found that AB appears to be more specific than chromomycine [4].
In group 1, the patients were treated for five weeks with daily doses of Fertibiol® (Nurilia, France, www.nurilia.com), a potent antioxidant complex containing ubiquinol as coenzyme Q10, Vitamin E (12mg), N-acetyl cysteine (200mg), Carnitine tartrate (134mg), asthaxanthine (4.3mg), all of the group B vitamins, and chelated Zinc (15mg). This was followed by treatment with Condensyl® (2 capsules per day) for at least 4 months (Nurilia, France; Parthenogen, Switzerland), which facilitates sperm maturation and correction of nuclear tertiary structure by supplying factors necessary for the 1-Carbon cycle: group B vitamins, bio available zinc, betalains and quercetin of natural origin and N-acetylcysteine [5,34]. This sequential treatment was designed after preliminary studies were Fertibiol was given alone. We observed that, in some patients, the treatment induced a strong decrease in sperm numeration. This decrease was reversible after arrest of the treatment.
Patients in Group 2 took only 2 capsules per day of Condensyl® during 4 months. The patients in group 3 took no supplements.
SDI and DFI were measured at the end of the treatment by a blinded analyst. Then the choice of the ART procedure was decided at the discretion of the gynecologist and the urologist post consultation.
Statistics: DFI and SDI values were compared before and after treatment using the Wilcoxon test. The differences (Δ) observed between the experimental groups before and after treatment were compared via the Mann-Whitney test. Chi square test was used for the pregnancy measures.
Results
Motility, sperm count and viability moved only marginally with treatments. The only difference was observed for the sequential treatment that decreased the numeration from 112 to 103 million sperm cells in the total ejaculate (p=0.01).
The changes in DFI and SDI values are depicted in Table 1. Baseline DFI was very high in all cases, with a mean of >20%. It improved significantly, with a reduction from 30.0 to 20.9% in Group 1 (p=0.001) and from 24.6 to 20% in Group 2 (p=0.003). All of the baseline SDI values were above the critical threshold of 25%. SDI improved to a lesser extent, from 39% to 35% in Group 1 (p<0.01) and from 42% to 35% in Group 2 (p=0.001). There were no changes in DFI (+1%) and SDI (+2%) in the control group.
Group
N
Paternal Age (yy)
DFI
SDI
Pre Rx
Post Rx
Δ (p*)
Pre Rx
Post Rx
Δ (p)
1. Fertibiol® + Condensyl®
151
37.6
(6.8)
30.3
(13.0)
20.9
(14.2)
-31%
(0.001)
39.0
(12.0)
35.0
(13.9)
-10%
(<0.01)
(p**)
<0.01
<0.01
2. Condensyl®
69
37.6
(5.9)
24.6
(12.4)
20
(12.7)
-19%
(0.003)
41.7
(9.7)
35.0
(15.0)
-16%
(0.001)
(p**)
<0.01
<0.01
3. Controls
84
37.8
(4.5)
30.9
(34.4)
31,2
(34.3)
+1%
(0.322)
30.5
(6.4)
31.1
(3.5)
+2%
(0.148)
Mean values (+/- standard deviation); Δ = differences observed between the experimental groups before and after treatment; p* = p value vs pre Rx (Wilcoxon test); p** = p value vs controls (Mann Whitney test).
Table 1: DFI and SDI changes before and after the treatment.
The pregnancy outcomes are reported in Table 2. Both treatments significantly improved the clinical pregnancy rates, from 27.4% in the control group to 53.6% in Group 1 and 50.7% in Group 2. A high percentage of the pregnancies, 22% in Group 1 and 28% in Group 2, occurred spontaneously, i.e. before performing the planned ART cycle, whereas no spontaneous pregnancies occurred in the control group.
Groups
Pregnancies
Births
Treatment
N
Mother Age (yy)
N (%)
p
Miscarr.
N (%)
Lost to F-U
N (%)
N (%)
p
1. Fertibiol® + Condensyl®
151
34
(4.3)
81
(53.6%)
<0.001
12 (1 TA)
(14.8%)
4
(4.9%)
65
(43.0%)
<0.001
2. Condensyl®®
69
36.1
(4.1)
35
(50.7%)
0.003
3 (1 TA)
(8.6%)
2
(5.7%)
29
(42.0%)
0.003
3. Controls
83
34.6
(5.4)
23
(27.4%)
5 (1 TA)
(21.7%)
0
(0.0%)
18
(21.4%)
p = p value vs controls (Chi 2 test).
Table 2: Pregnancy outcomes according to the treatment regime.
Discussion
In the absence of therapeutic intervention, DFI and SDI are much more stable over time than are the classical semen parameters of count, motility and morphology [5,38]. Our study demonstrates that antioxidant nutritional support resulted in a significant decrease in both DFI and SDI. No variation was observed in the control group. This confirms that stimulating endogenous generation of antioxidants, i.e. glutathione and perhaps to a lesser extent hypotaurine, through the cystathionine beta synthase pathway (CBS), can not only prevent DNA decays in terms of fragmentation, but also prevents nuclear decondensation. It simultaneously allows homocysteine recycling to methionine and then S-adenosylmethionine (SAM), which is involved in epigenetic modifications necessary for further embryonic development. SAM is also involved in the synthesis of spermine and spermidine, multifunctional compounds that control intracellular pH and volume, with an impact on DNA stability and chromatinmediated regulation of gene expression. Guanine is the DNA base the most sensitive to oxidation and a strong correlation has been established between the 8-oxo guanosine content and Sperm DNA fragmentation [39]. The importance of 8-oxo guanosine in relation to the oxidation of DNA bases must not be overlooked. If left unrepaired, 8-oxoG affects the methylation of adjacent cytosine (CpG, the phosphodiester bond between cytosine and guanine): these CpG sites in DNA represent mutational hotspots [40-41]. Telomeres, with repeats of TTAGGG, have a high G content. Critically, short telomeres are associated with sperm DNA fragmentation [42]. Telomere shortening induces defects in meiosis, fertilization and embryo development and may thus lead to infertility [43]. Telomere dysfunction is one of the epicenters in reproductive aging. 8-oxo guanosine may also affect codons for the sulfur-containing amino acid cysteine (UGU, UGC), which is involved in the synthesis of glutathione and hypotaurine, as well as the codon for methionine (AUG): methionine is the universal methylating agent, through S-adenosyl methionine. These observations strongly suggest a link between reactive oxygen species (ROS) decays, paternal age, DNA methylation, genetic alterations and carcinogenesis.
The antioxidant treatments seem to decrease the rate of miscarriage (14.8% and 8.6% vs 21.7%), which is in agreement with a link between miscarriage, a paternal effect via DNA fragmentation, and methylation [15,44] However the numbers in this study are too small for any accurate statement to be suggested. The effect of 1-C cycle sustainer towards methylation should not be overlooked, Condensyl decreases SDI but, in some case it remains high as observed by Dattilo et al. [34]. It is now of common knowledge that sperm DNA methylation affects fertility and further on the quality of the conceptus through epigenetic mechanism [31,45]. The occurrence of a high rate of spontaneous pregnancies in the treated groups (22% and 28% respectively in Group 1 and 2) strongly suggests an improvement in sperm quality.
The pre-treatment with strong antioxidants before one-carbon cycle support did not improve the outcomes as compared to onecarbon cycle support alone. However, it is not obvious that a sequential treatment with first a strong reducing shock is useless, especially if we consider that DFI can pass easily the threshold of 50% and over [46]. A strong “reducing shock” may probably help in reducing the treatment time; especially in patients with a very high DFI (It can reach 94% according to [46]. Subsequent treatment to stimulate the one-carbon cycle may be necessary/ useful in order to padlock protamines, and thus reconstitute nuclear tertiary structure to facilitate a correct epigenetic process. One must not overlook that ICSI is not a “miracle” technique [47] and that improvements can be made upstream ART techniques.
In conclusion, the present study demonstrates that a nutritional support capable of activating and sustaining the 1-Carbon cycle is effective in decreasing the DFI and the SDI and in improving the pregnancy outcome in ART couples carrying a male factor. However, these findings are to be taken with caution due to the small sample size and none truly randomized nature of the study. Future investigations based on a larger sample and on stricter randomization and blinding procedures are needed.
Acknowledgments
This work was not sponsored, received no financial support, and all of the patients paid for their treatments. The authors declare no conflict of interest.
EA was responsible for clinical andrology and urology (American Hospital, Paris), DC and MC were responsible for clinical gynecology and ART (Clinique de la Muette Paris), YM was responsible for all biological aspects and authored the paper.
Many thanks to Dr Kay Elder for review of the final manuscript and to Isabelle Loudcher for statistical analyses.
References
- Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980; 210: 1131-1133.
- Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006; 103: 9601-9606.
- Sergerie M, Laforest G, Boulanger K, Bissonnette F, Bleau G. Longitudinal study of sperm DNA fragmentation as measured by terminal uridine nick end-labelling assay. Hum Reprod. 2005; 20: 1921-1927.
- Belloc S, Benkhalifa M, Junca AM, Dumont M, Bacrie PC, Ménézo Y. Paternal age and sperm DNA decay: discrepancy between chromomycin and aniline blue staining. Reprod Biomed Online. 2009; 19: 264-269.
- Menezo Y, Evenson D, Cohen M, Dale B. Effect of antioxidants on sperm genetic damage. Adv Exp Med Biol. 2014; 791: 173-189.
- Menezo YJR, Russo G, Tosti E, El Mouatassim S & Benkhalifa M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. J Assist Reprod Genet. 2007; 24: 513–520.
- El-Mouatassim S, Bilotto S, Russo GL, Tosti E, Menezo Y. APEX/Ref-1 (apurinic/apyrimidic endonuclease DNA-repair gene) expression in human and ascidian (Ciona intestinalis) gametes and embryos. Mol Hum Reprod. 2007; 13: 549-556.
- Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010; 18: 357-365.
- Ménézo YJ. Paternal and maternal factors in preimplantation embryogenesis: interaction with the biochemical environment. Reprod Biomed Online. 2006; 12: 616-621.
- Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008; 14: 243-258.
- Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011; 26: 1628-1640.
- Aitken RJ, Baker MA, De Iuliis GN, Nixon B. New insights into sperm physiology and pathology. Handb Exp Pharmacol. 2010; 198: 99-115.
- Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013; 11:154.
- Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014; 32: 1-17.
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012; 27: 2908-2917.
- Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008; 78: 761-772.
- Lopes S, Jurisicova A, Casper RF. Gamete-specific DNA fragmentation in unfertilized human oocytes after intracytoplasmic sperm injection. Hum Reprod. 1998; 13: 703-708.
- Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008; 89: 1183-1190.
- De Iuliis GN, Wingate JK, Koppers AJ, McLaughlin EA, Aitken RJ. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab. 2006; 91: 1968-1975.
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011; : CD007411.
- Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. Is ascorbate able to reduce disulfide bridges? A cautionary note. Nitric Oxide. 2008; 19: 252-258.
- Carrell DT. Contributions of spermatozoa to embryogenesis: assays to evaluate their genetic and epigenetic fitness. Reprod Biomed Online. 2008; 16: 474-484.
- Rousseaux S, Reynoird N, Escoffier E, Thevenon J, Caron C, Khochbin S. Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod Biomed Online. 2008; 16: 492-503. https://www.ncbi.nlm.nih.gov/pubmed/17425820
- Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007; 14: 418-421.
- Pfeifer H, Conrad M, Roethlein D, Kyriakopoulos A, Brielmeier M, Bornkamm GW, et al. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J. 2001; 15: 1236-1238.
- Ménézo Y, Entezami F, Lichtblau I, Belloc S, Cohen M, Dale B. Oxidative stress and fertility: incorrect assumptions and ineffective solutions? Zygote. 2014; 22: 80-90.
- Ménézo Y, Mares P, Cohen M, Brack M, Viville S et al. Autism, imprinting and epigenetic disorders: a metabolic syndrome linked to anomalies in homocysteine recycling starting in early life? J Assist Reprod Genet. 2011; 28:1143–1145.
- Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011; 77: 1088-1093.
- Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009; 26: 537-544.
- Padmanabhan N, Watson ED. Lessons from the one-carbon metabolism: passing it along to the next generation. Reprod Biomed Online. 2013; 27: 637-643.
- Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014; 10: e1004458.
- Junca A, Gonzalez Marti B, Tosti E, Cohen M, De la Fontaine D, Benkhalifa M, et al. Sperm nucleus decondensation, hyaluronic acid (HA) binding and oocyte activation capacity: different markers of sperm immaturity? Case reports. J Assist Reprod Genet. 2012; 29: 353-355.
- Singh K, Jaiswal D. One-carbon metabolism, spermatogenesis, and male infertility. Reprod Sci. 2013; 20: 622-630.
- Dattilo M, Cornet D, Amar E, Cohen M, Menezo Y. The importance of the one carbon cycle nutritional support in human male fertility: a preliminary clinical report. Reprod Biol Endocrinol. 2014; 12: 71.
- Guérin P, Ménézo Y. Hypotaurine and taurine in gamete and embryo environments: de novo synthesis via the cysteine sulfinic acid pathway in oviduct cells. Zygote. 1995; 3: 333-343.
- Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009; 91: 1801-1805.
- Hammadeh ME, Zeginiadov T, Rosenbaum P, Georg T, Schmidt W, Strehler E. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch Androl. 2001; 46: 99-104.
- Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002; 23: 25-43.
- Oger I, Da Cruz C, Panteix G, Menezo Y. Evaluating human sperm DNA integrity: relationship between 8-hydroxydeoxyguanosine quantification and the sperm chromatin structure assay. Zygote. 2003; 11: 367-371.
- Wachsman JT. DNA methylation and the association between genetic and epigenetic changes: relation to carcinogenesis. Mutat Res. 1997; 375: 1-8.
- Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008; 266: 6-11.
- Rodríguez S, Goyanes V, Segrelles E, Blasco M, Gosálvez J, Fernández JL. Critically short telomeres are associated with sperm DNA fragmentation. Fertil Steril. 2005; 84: 843-845.
- Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009; 21: 10-14.
- Anderson D, Schmid TE, Baumgartner A. Male-mediated developmental toxicity. Asian J Androl. 2014; 16: 81-88.
- Jenkins TG, Carrell DT. The sperm epigenome and potential implications for the developing embryo. Reproduction. 2012; 143: 727-734.
- Evenson DP, Brannian J , Hansen K , Kasperson K, Christianson J. Relationship between sperm DNA fragmentation , age of donors and patients, and children with psychic disorders, Abstr ASRM meeting Hawaii. 2014.
- Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015; 313: 255-263.
Citation: Amar E, Cornet D, Cohen M and Ménézo Y. Treatment for High Levels of Sperm DNA Fragmentation and Nuclear Decondensation: Sequential Treatment with a Potent Antioxidant Followed by Stimulation of the One-Carbon Cycle vs One-Carbon Cycle Back-up Alone. Austin J Reprod Med Infertil. 2015;2(1): 1006. ISSN:2471-0393