
Research Article
Austin J Reprod Med Infertil. 2015;2(2): 1011.
Clinical Evidence for the Importance of 1-Carbon Cycle Support in Subfertile Couples
Dominique Cornet¹, Edouard Amar², Marc Cohen³ and Yves Ménézo4*
¹Clinique de la Muette , Rue Nicolo, 75016 Paris, France
²Cabinet Médical d’Andrologie, 17 avenue Victor Hugo, 75016 Paris, France
³Clinique Natecia, Avenue Rockefeller, Lyon France
4Laboratoire Clément, 17 avenue d’Eylau, 75016 Paris and London Fertility associates, 104 Harley street, London WIG7JD, UK
*Corresponding author: Yves Ménézo, 4Laboratoire Clément, 17 avenue d’Eylau, 75016 Paris and London Fertility associates, 104 Harley street, London WIG7JD, UK
Received: March 16, 2015; Accepted: May 10, 2015; Published: May 29, 2015
Abstract
This study assesses the effect of nutritional support directed towards the 1-C cycle on the fertility of male and female patients attending an ART program. In a first group, female partners of couples having failed at least one ART attempt were administered 1-C nutritional support prior to a further ART cycle. A second group comprised couples who had failed at least 2 assisted reproductive technology (ART) attempts, with male partners having elevated DNA fragmentation index (DFI) or nuclear decondensation index (SDI). The treatment consisted of compounds that play a major role in the 1-C cycle: B vitamins, chelated zinc and N-acetyl cysteine.
The first group of 100 treated female patients achieved a clinical pregnancy rate (PR) of 45%, with a high rate of spontaneous conception before ART. This was significantly higher (p=0.0001) than the PR observed in the control group of 73 patients, with PR =13.7%. When the male partners alone (95) were treated, a significant decrease was observed for DFI and SDI vs. control (84), and this was associated with a significant increase in the delivery rates (47.4% vs. 21.4%, p=0.001). Nutritional support of the 1-C cycle improves male and female fertility; this should not be overlooked, especially for women entering ART programs.
Keywords: ART; SDI; DFI; Oxidative stress; DNA
Introduction
Oxidative stress (OS) represents a hazard to reproductive processes. The impact of OS on male fertility, i.e. on sperm quality, has been recognized since the pioneering works of Evenson et al. [1] in the early 1980’s, and it now appears that damage caused by OS is shared equally between male and female [2]. Evidence indicates that damage can be attributed to roughly one-third of maternal origin, one-third paternal, and one-third shared between both partners. A better appreciation of sperm quality through estimation of DNA integrity is now available: fragmentation (DNA fragmentation index, DFI) and sperm nuclear decondensation index (SDI), or high DNA stainability (HDS). DFI and SDI correlate poorly with the classical WHO sperm parameters of count, motility and morphology, and without treatment are relatively stable over time [3-6]. The DFI increases considerably with age [3,5,6], but SDI decreases slightly, indicating an age-related weakened defense against reactive oxygen species [6]. Damage of paternal origin has multiple sources for ROS-linked decays that originate partly, but not entirely from the spermatozoon itself, i.e. the mitochondria [7]. More than 10 base oxidation products [8], DNA adducts [9], and DNA strand breaks [1], as well as some features of nuclear decondensation are ROS-derived. In the female partner, adduct formation and DNA base oxidation add to age-associated meiotic defects observed in oocytes from older patients; in combination with aberrant response to DNA damage and telomere recombination, all of these effects can lead to infertility or early miscarriage [10]. In theory, the oocyte/zygote can repair these chemical insults to some extent [8,11], but the capacity for repair is finite, and decreases with age [12]. When the oocytes’ capacity for DNA repair is overwhelmed, a process of “tolerance” allows mutations that lead to miscarriages and other later pathologies [13- 14]. Recent observations in animals [15] have emphasized potential risks to postnatal development after performing micro-injection with highly fragmented DNA. It is tempting to try to reduce or avoid DNA damage related to ROS through consumption of anti-oxidants, and taking vitamins A, E and C, often coupled with selenium, seems an attractive proposition. Vitamins C and E can be either anti- or pro-oxidant. Equilibrium between oxidation and reduction status must be maintained over time. Vitamin C opens disulfide bridges of glutathione and protamines [16], leading to nuclear decondensation in the spermatozoon: oxidized glutathione is essential for padlocking and cross-linking protamines [17]. DNA decondensation induces chromosomal anomalies, and thus is deleterious for early development [18-19]. We recently observed [20] a process of sperm decondensation after treatment with mixtures of selenium and vitamins A, C, and E. The practice of ingesting large ‘blind’ doses of anti-oxidants is highly questionable [21], and has been shown to be poorly efficient, at least in women [22]: the final outcome, i.e. ongoing pregnancies and deliveries, are seldom taken into account. The fact that there is a strong correlation between oxidative stress and DNA methylation is no longer in question, and this has a further subsequent impact on transmitted diseases [23-25]. There is a clear biochemical explanation behind this concept, as seen in Figure 1. Oxidative stress has an influence on the one carbon (1-C) cycle, which is involved in homocysteine recycling with formation of methionine, necessary for DNA methylation. Through synthesis of cystine, products of the 1-C cycle include two major endogenous antioxidants: hypotaurine [26] present in the environment of oocytes, sperm and embryos, and glutathione. Ovarian stimulation has a negative impact on homocysteine metabolism [27]: homocysteine competes with the same oocyte receptor for methionine, and thus inhibits methylation reactions that are necessary for imprinting [28]. The cysteine beta synthase (CBS) pathway allows recycling of homocysteine to some extent, but this pathway is not expressed in the human oocyte [29]. Therefore, until the time of genomic activation on the third day postfertilization, the human zygote genome is poorly defended from the negative impact of homocysteine on supplies of methionine that are required to maintain methylation, with a downstream effect on imprinting. In addition, female and male reproductive physiology is now at risk from plastic-derived endocrine disruptors present at high levels in our current environment. This study was conducted in order to investigate the impact of nutritional support for the 1-C cycle in female and male partners consulting for ART.
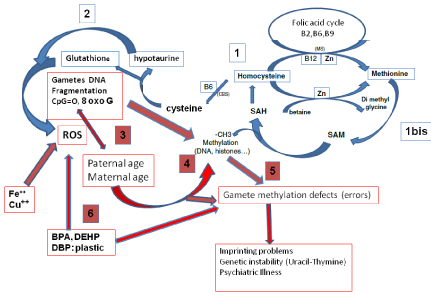
Figure 1: Interrelations between oxidative stress and methylation processes
1- Correct recycling of Homocysteine allows generation of Cysteine and
methionine which allows correct processes of methylation (1bis). 2- Correct
generation of cysteine allows synthesis of Hypotaurine and glutathione, two
potent inhibitors of ROS. 3- Generation of ROS induces DNA fragmentation.
Advanced age decreases the ability to control ROS-linked decays. 4-High
levels of homocysteine inhibit methylation processes in sperm physiology
(methylation of the membrane lipids) and in sperm DNA. 5 - DNA methylation
defects, whether or not linked to imprinting may result in negative
transgenerational health problems. Unrepaired 8 oxoG leads to aberrant
methylation at CpG sites, impair transcription and may affect telomere length.
6- Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) worsen all
the negative steps.
Materials and Methods
All of the studies were performed at a French private clinic that is not required to have, and does not have, a reference Institutional Review Board; however the protocols were conducted in full conformity with the current revision of the Declaration of Helsinki. The protocol was carried out during the period January - August 2013, in an ART center performing 1800 IVF/ICSI cycles per year. All subjects gave their informed consent to participate in the study. Follow-up continued until the time of data collection in December 2013, and all pregnancies were then monitored and followed to their outcome.
Female patients whose male partners had normal WHO parameters
All of the female patients admitted to the program had primary infertility of at least 3 years’ duration, with a minimum of one previous failed ART treatment cycle (mean 2.0). All of the women were <43 years of age (as regulated by French law), and had no pregnancy with a previous partner. The male partners had sperm parameters above the threshold for normality, as determined by the “WHO laboratory manual for the examination and processing of human semen” (2010). Female partner serum FSH and AMH (anti-Mullerian hormone) levels were measured on Day 3 of the cycle, with no indication of ovarian failure in either the control or the study group (AMH >1.5μG/L and FSH <9 UI/L); FSH levels were slightly higher in the control group (7.9 vs 6.2, p=0.02) but AMH was lower in the treatment group (2.3 vs 2.42, p= 0.12, respectively). All values were considered to be in the normal range. The incidence of polycystic ovarian syndrome (PCOs) and endometriosis was close to 50% in both groups, with no statistical difference: these two pathologies are significantly linked to oxidative stress. Table 1 illustrates the background data obtained for the two groups.
N (Patients)
FSH (SD)
AMH (SD)
Age (SD)
Maternal
Paternal
Treated
100
6.2 (1.7)
2.3 (1.5)
32.5 (4.1)
33.7 (4.0)
Control
73
7.9 (3.2)
2.4 (2.6)
34.7 (5.3)
36.8 (5.8)
p
0.02
0.12
NS
NS
Table 1: Sample characteristics of female patients treated vs control (Mann Whitney); Treated: 19 endometriosis (19%), 30 PCOS (30%); Control: 9 endometriosis (12.3%), 19 PCOS (27%).
Female patients in the treatment group received one tablet of ProcreliaTM Woman (Parthenogen Lugano, CH, Nurilia, Lyon France) per day, starting three months before their ART protocol. As shown in Table 2, Procrelia™ Woman contains compounds of the onecarbon cycle, vitamins of group B, chelated Zn , N-acetyl cysteine, weak antioxidants and fish oil. To ensure activity, Zn must be added in a chelated form (www.efsa.europa.eu/, European Food Safety authority: EFSA-Q-2005-035, EFSA-Q-2005-133, EFSA-Q-2005-034, EFSA-Q-2005-038, EFSA-Q-2005-166, EFSA-Q-2005-033, EFSA-Q-2005-132, EFSA-Q-2005-036, EFSAQ-2005-130).
Compound
mg (%GDA)
N-acetylcysteine
250
Zinc
15 (150%)
Vitamin E
10 (83.2%)
Vitamin B3
16.0 (100%)
Vitamin B6
1.4 (100%)
Vitamin B2
1,40 (100%)
Vitamin B9
0.4 (200%)
Vitamin B12
2.5x10-3 (100%)
Prickle pear extract
270
Betalaine (from beetroot)
40
Procrelia WomenTM contains the same constituents + 40mG of Fish oil.
Table 2: Supplement formulations (mG,% Guideline Daily Amounts)/ Procrelia Man™.
Male patients with abnormal (elevated) SDI and subnormal DFI whose female partner had no detected anomalies
The duration of primary infertility in these couples was at least 3 years, with at least one failed ART (IVF/ ICSI) attempt, and no pregnancy with a previous partner. Oligoasthenospermic patients were excluded. Total sperm count and viability (eosin-nigrosin on at least 200 cells) in the ejaculates were performed according to WHO (2010) guidelines in an accredited laboratory (ISO 15189). All total counts in the ejaculates were above the WHO threshold for infertility: treated, 48.7 million/ml (SD: 43.8); control, 46.8 (29.7). Motility was greater than 15%: treated patients, 21.1 % (SD: 6.2); control, 17.6 (SD: 6.4), NS. None of the female partners had detectable pathologies: normal ovulatory profile, endocrinological parameters (FSH< 8UI/L, estradiol<40μg/L) and ovarian reserve (AMH>2μg/l). The female partners were <43 yrs of age, as recommended by the French Biomedicine Agency (moreover, patients > 43 yrs are not reimbursed by the Social security). This pilot study was not randomized with a placebo group; the control group consisted entirely of male patients who received no complementary treatment during their ART cycles. The male partners were under consultation for primary infertility (duration >3 yrs) with previous IVF/ICSI attempts, and no pregnancies with a previous partner. All had been tested for testicular volume and varicocele by testicular palpation and sonography, and testosterone levels were in the normal range. Sperm DFI was measured via flow cytometry terminal deoxynucleotidyl transferased UTP nick end labeling (Tunel) assay [30]. The SDI was measured by microscopy of 200 cells using aniline blue (AB) [31], since it has been shown that AB appears to be more specific than chromomycine [5] in detecting DNA fragmentation. Values for sperm DNA structure are presented in Table 3.
N (patients)
Pregnancies
Miscarriages
Deliveries (%)
Treated
100
451 (45%)
5 (11%)
40 (40%)
Control
73
102 (13.7%)
2 (20%)
8 (10.9%)
p
0.0001
Statistics: Chi square;
*1: distribution of the pregnancies: 4/16 IVF (In vitro fertilization), 11/17 ICSI(Intra- cytoplasmic sperm injection), 30 spontaneous. No Artificial insemination with Husband (AIH)
*2: distribution of the pregnancies: 3/9 IVFs, 3/51 AIHs, 4/28 ICSIs, 0/9 frozen embryo transfers
Table 3: Pregnancies and deliveries: women treated only, male partner “normal”, treatment vs. Control.
The patients took one tablet of Procrelia Man (™) per day for a period of 4 months. SDI and DFI were re-assessed after treatment. The control group received no supplement and was monitored after 20 wks. The composition of Procrelia Man is the same as Procrelia Woman, with fish oil extract (see Table 2).
SDI and DFI were measured at the end of the treatment period via blinded analysis. The choice of ART procedure was then decided postconsultation at the discretion of the gynecologist and the urologist.
Statistics
This investigation was carried out as a pilot study: the Mann– Whitney test (2 independent groups) was used for continuous variables. Basic descriptive statistics (mean, range and standard deviation) were determined as appropriate, and statistical analysis was performed using IBM SPSS statistical software. The Chi2 test (test of homogeneity) was used to estimate the independence of two categorical variables, and for comparison of percentages.
Results
Female patients whose male partners had normal WHO parameters (Table 3)
The results show a highly significant increase in pregnancy rate for the treated group: 45% vs. 13.7% for the control group, p<0.001. The most significant observation is a very high spontaneous pregnancy rate prior to starting a proposed ART procedure in the treatment group: 30/100 per patients and 2/3rd of the pregnancies, vs. none in the control group. The number of miscarriages was insufficient for statistical analysis (11% vs. 20% in the control group).
Male patients with abnormal (elevated) SDI and subnormal DFI whose female partner had no detected anomalies (Tables 4 and 5)
Treatment modified sperm count and motility only marginally (count, +5.1%, control, +6.8%, NS); motility (+12%, control: -1.6%, p=0.01). As shown in Table 4, a significant decrease was observed for DFI (-36.3%, p=0.001) and SDI (-29.7%, p=0.32). The pregnancy rates were also significantly increased in the treated group: 56% vs. 27.4%, p = 0.001 (Table 5). The percentage of spontaneous pregnancies, before starting an ART attempt is also high in this group (8/95: 8.4% of the patients and 8/49=16.3% of all of the pregnancies). It was impossible to determine an effect on miscarriage due to the small numbers (Treated: 8%, control: 21.7%).
N (Patients)
Pregnancies
Miscarriages
Deliveries (%)
Treated
100
45*1(45%)
5 (11%)
40 (40%)
Control
73
10*2(13.7%)
2 (20%)
8 (10.9%
p
0.0001
Statistics: Chi square;
*1: 4/16 IVF ( In vitro fertilization), 11/17 ICSI(Intra- cytoplasmic sperm injection), 30 spontaneous. No Artificial insemination with Husband (AIH).
*2: 3/9 IVFs, 3/51 AIHs, 4/28 ICSIs, 0/9 frozen embryo transfers.
Table 4: Pregnancies and deliveries, female partners only treated, treatment vs control.
N
MATERNAL AGE
Pregnancies
Deliveries
Treated
95
32.9 (3.8)
49 (56%)*1
45 (47.4%)
p= 0.001
Control
84
34.6 (5.4)
23 (27.4%)*²
18 (21.4%)
*1: 8 Spontaneous, 11/25 IVFs (44%), 12/30 ICSIs (40%), 11/20 AIHs (55%), 8/12 F/T embryo transfers (66%).
*² : 3/22 IVFs (13.6%), 13/29 ICSIs (44.8%), 6/15 AIHs (40%),1/18 F/T embryo transfers (5.5%).
Table 5: Pregnancies and deliveries in treated and control group (male treatment with Procrelia Man) Female Partner normal.
Discussion and Conclusions
The present study reports clinical pregnancy (CPR) and live birth rates (LBR) of 45% and 40% respectively in female patients treated with supplements to support the 1-C cycle. Of particular note is the fact that two-thirds (30/45) of the pregnancies were spontaneous, with conception occurring during the run-up to a planned ART cycle. This means that the oocytes have decreased their DNA damage and have also increased their DNA repair capacity. This perspective of the female contribution to a couple’s infertility supports the observations published by Dattilo et al. [32]. When the female gamete is compromised, treating male partners alone with supplements involved in 1-C support leads to an immediate dramatic decrease in the LBR: this may indicate that improvements in sperm quality may be of little help in the presence of an added female factor. The same symmetrical observation can be made when males are treated; the CPR and LBR are increased (but to a lesser extent when compared to that observed for women). Although it is possible to determine the mechanisms behind the effects of treatment on sperm DNA structure, this is not the case in the female. As expected from published data [2] the female gamete is at risk from decays linked to oxidative stress. The 1-C cycle recycles homocysteine, and this represents a major effectors in regulation of female fertility. Homocysteine lies at the crossroads of the problem, especially in the case of the oocyte [27,33]. This feature is of particular concern in view of the fact that environmental conditions, especially plastic-derived endocrine disruptors (a) have estrogen-like compounds (see figure 1) and (b) are found in the urine of patients entering ART programs [34]. Ovarian stimulation increases in complexity with increasing levels of urinary BPA, associated with decreasing ovarian response; (c) Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) induce Epigenetic Transgenerational Inheritance in animals through an inhibitory effect on methylation processes, inducing epimutations (d) these compounds induce oxidative stress and mitochondrial dysfunction.
All of the oxidative stress markers are elevated in PCOs patients, who may also suffer effects on methylation via an increase in circulating homocysteine [35]; levels of circulating glutathione, (pivotal molecule in protection against ROS) are also decreased. This strongly suggests that oxidative stress may participate in the pathophysiology of PCOs and endometriosis [36], with a negative effect on oocyte quality and subsequently on embryo development and then on rates of miscarriage. Clearly, oxidative damage and methylation defects are relevant clinical targets that can be positively influenced by tailored nutritional supplementation: this is a feature that should not be overlooked in assisted reproduction technology, especially in consideration of the high rates of spontaneous conception revealed in this study. This type of nutritional support seems at least as important in women as in men. The negative impact of endocrine disruptors present in the environment with an effect on epigenetic reprogramming and reproduction is a further reason for positive consideration of these supplements. In the absence of therapeutic intervention, DFI and SDI are much more stable over time than are the classical semen parameters of count, motility and morphology [4,5,6,37]. Our study confirms that antioxidant nutritional support resulted in a significant decrease in both DFI and SDI [30]. No variation was observed in the control group. This confirms that stimulating endogenous generation of antioxidants, i.e. glutathione and perhaps to a lesser extent hypotaurine, through the cystathionine beta synthase pathway (CBS) not only prevents DNA decays in terms of fragmentation, but also prevents nuclear decondensation. The protective antioxidant activity protects against oxidation of guanine, the most sensitive base. 8-oxo guanosine formation correlates with DNA fragmentation [31]. Telomeres, with repeats of TTAGGG, have a high G content. Critically, short telomeres are associated with sperm DNA fragmentation [38]. Telomere shortening induces defects in meiosis, fertilization and embryo development and may thus lead to infertility. Telomere dysfunction is one of the epicenters in reproductive aging [10].
The One-carbon cycle simultaneously allows homocysteine recycling to methionine and then S-adenosylmethionine (SAM), which is involved in epigenetic modifications necessary for further embryonic development. SAM is also involved in the synthesis of spermine and spermidine, both with a positive impact on DNA stability and chromatin-mediated regulation of gene expression. The effect of the 1-C cycle in sustaining methylation should not be overlooked: it is now common knowledge that sperm DNA methylation affects fertility, and further the quality of the conceptus through epigenetic mechanisms [39,40]. All of these observations reinforce the suggested link between reactive oxygen species (ROS) decays, paternal age, DNA methylation, some genetic alterations, psychic disorders in children [23, 29 40, 41] and in some cases carcinogenesis. One must not overlook the fact that ICSI is not a “miracle” techniques [42], and that upstream improvements can be made in ART techniques.
In conclusion, the present study demonstrates that nutritional support capable of activating and sustaining the 1-Carbon cycle is effective in improving the pregnancy outcome in couples consulting for infertility. It could also be considered as a form of protection against some of the potential risks for the children. None of these considerations should be overlooked, especially with regard to the increasing pollution by endocrine disruptors, true inhibitors of methylation, which induce epigenetic transgenerational inheritance of reproductive disease and sperm epimutations [43].
References
- Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980; 210: 1131-1133.
- Lopes S, Jurisicova A, Casper RF. Gamete-specific DNA fragmentation in unfertilized human oocytes after intracytoplasmic sperm injection. Hum Reprod. 1998; 13: 703-708.
- Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006; 103: 9601-9606.
- Sergerie M, Laforest G, Boulanger K, Bissonnette F, Bleau G. Longitudinal study of sperm DNA fragmentation as measured by terminal uridine nick end-labelling assay. Hum Reprod. 2005; 20: 1921-1927.
- Belloc S, Benkhalifa M, Junca AM, Dumont M, Bacrie PC, Ménézo Y. Paternal age and sperm DNA decay: discrepancy between chromomycin and aniline blue staining. Reprod Biomed Online. 2009; 19: 264-269.
- Menezo Y, Evenson D, Cohen M, Dale B. Effect of antioxidants on sperm genetic damage. Adv Exp Med Biol. 2014; 791: 173-189.
- De Iuliis GN, Wingate JK, Koppers AJ, McLaughlin EA, Aitken RJ. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab. 2006; 91: 1968-1975.
- Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010; 18: 357-365.
- Badouard C, Ménézo Y, Panteix G, Ravanat JL, Douki T, Cadet J, et al. Determination of new types of DNA lesions in human sperm. Zygote. 2008; 16: 9-13.
- Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009; 21: 10-14.
- Menezo Y Jr, Russo G, Tosti E, El Mouatassim S, Benkhalifa M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. J Assist Reprod Genet. 2007; 24: 513-520.
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004; 13: 2263-2278.
- Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008; 14: 243-258.
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012; 27: 2908-2917.
- Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008; 78: 761-772.
- Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. Is ascorbate able to reduce disulfide bridges? A cautionary note. Nitric Oxide. 2008; 19: 252-258.
- Pfeifer H, Conrad M, Roethlein D, Kyriakopoulos A, Brielmeier M, Bornkamm GW, et al. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J. 2001; 15: 1236-1238.
- Carrell DT. Contributions of spermatozoa to embryogenesis: assays to evaluate their genetic and epigenetic fitness. Reprod Biomed Online. 2008; 16: 474-484.
- Rousseaux S, Reynoird N, Escoffier E, Thevenon J, Caron C, Khochbin S. Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod Biomed Online. 2008; 16: 492-503.
- Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007; 14: 418-421.
- Ménézo Y, Entezami F, Lichtblau I, Belloc S, Cohen M, Dale B. Oxidative stress and fertility: incorrect assumptions and ineffective solutions? Zygote. 2014; 22: 80-90.
- Showell MG, Brown J, Clarke J, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2013; 8: CD007807.
- Menezo Y, Mares P, Cohen M,Brack M, Viville S, ElderK. Autism, imprinting and epigenetic disorders: a metabolic syndrome linked to anomalies in homocysteine recycling starting in early life? J Assist Reprod Genet. 2011; 28:1143–1145.
- Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011; 77: 1088-1093.
- Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009; 26: 537-544.
- Guérin P, Ménézo Y. Hypotaurine and taurine in gamete and embryo environments: de novo synthesis via the cysteine sulfinic acid pathway in oviduct cells. Zygote. 1995; 3: 333-343.
- Boxmeer JC, Steegers-Theunissen RP, Lindemans J, Wildhagen MF, Martini E, Steegers EA, et al. Homocysteine metabolism in the pre-ovulatory follicle during ovarian stimulation. Hum Reprod. 2008; 23: 2570-2576.
- Menezo Y, Khatchadourian C, Gharib A, Hamidi J, Greenland T, Sarda N. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci. 1989; 44: 1601-1609.
- Ménézo Y, Lichtblau I, Elder K. New insights into human pre-implantation metabolism in vivo and in vitro. J Assist Reprod Genet. 2013; 30: 293-303.
- Amar E, Cornet D, Cohen M & Menezo Y. Treatment for high levels of sperm DNA fragmentation and nuclear decondensation: sequential treatment with a potent antioxidant followed by stimulation of the one-Carbon cycle vs one-Carbon cycle back-up alone. Austin J Reprod Med Infertil in press. 2015.
- Oger I, Da Cruz C, Panteix G, Menezo Y. Evaluating human sperm DNA integrity: relationship between 8-hydroxydeoxyguanosine quantification and the sperm chromatin structure assay. Zygote. 2003; 11: 367-371.
- Dattilo M, Cornet D, Amar E, Cohen M, Menezo Y. The importance of the one carbon cycle nutritional support in human male fertility: a preliminary clinical report. Reprod Biol Endocrinol. 2014; 12: 71.
- Ebisch IM, Peters WH, Thomas CM, Wetzels AM, Peer PG, Steegers-Theunissen RP. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod. 2006; 21: 1725-1733.
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010; 33: 385-393.
- Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013; 19: 268-288.
- Rosa e Silva JC, do Amara VF, Mendonça JL, Rosa e Silva AC, Nakao LS, Poli Neto OB, et al. Serum markers of oxidative stress and endometriosis. Clin Exp Obstet Gynecol. 2014; 41: 371-374.
- Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002; 23: 25-43.
- Rodríguez S, Goyanes V, Segrelles E, Blasco M, Gosálvez J, Fernández JL. Critically short telomeres are associated with sperm DNA fragmentation. Fertil Steril. 2005; 84: 843-845.
- Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009; 26: 537-544.
- Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014; 10: e1004458.
- Evenson DP, Brannian J , Hansen K , Kasperson K, Christianson J. Relationship between sperm DNA fragmentation , age of donors and patients, and children with psychic disorders, Abstr ASRM meeting Hawaii. 2014.
- Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015; 313: 255-263.
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013; 8: e55387.