
Research Article
Austin J Reprod Med Infertil. 2015;2(2): 1012.
Genetic Diagnosis in Non-Obstructive Azoospermic Tunisian Men
Wajih Hammami1,2, Olfa Kilani1,3, Mariem Ben Khelifa1,2, Wiem Ayed1,3, Abderrezzak Bouzouita3,5, Fethi Zhioua3,4, Sonia Abdelhak² and Ahlem Amouri1,2,3*
¹Department of Histology and Cytogenetics, Institut Pasteur de Tunis, Tunisia
²University of Tunis El Manar, LR11IPT05 Laboratory of Biomedical Genomics and Oncogenetics, Institut Pasteur de Tunis, Tunisia
³University of Tunis El Manar, Faculty of Medicine of Tunis, Tunisia
4Department of Obstetrics and Gynecology, Aziza Othmana, University Hospital, Tunis, Tunisia
5Department of Urology, Charles-Nicolle University Hospital, Medical University of Tunis, Tunisia
*Corresponding author: Ahlem Amouri, Department of Histology and Cytogenetics, Institut Pasteur de Tunis 13, Place Pasteur de Tunis, Tunisia
Received: March 05, 2015; Accepted: May 10, 2015; Published: May 20, 2015
Abstract
Purpose: Male infertility is the cause in half of all childless partnerships. Numerous factors contribute to male infertility, including chromosomal aberration and Yq microdeletions. We therefore aimed to evaluate the prevalence of genetic abnormalities among non-obstructive azoospermic (NOA) Tunisian men referred for routine cytogenetic analysis to the department of cytogenetics of the Pasteur institute of Tunis.
Methods: Karyotype analyses were performed on peripheral blood lymphocytes using R-banding for 401 NOA. Molecular diagnosis of classic Yq microdeletions was performed in 90 NOA with normal karyotypes by two multiplex PCRs using six STS markers (Sequence-Tagged Site) recommended by the EAA/EMQN (European Academy of Andrology / the European Molecular Genetics Quality Network).
Results: The overall incidence of chromosomal abnormalities was 12.22% (49/401). Out of the 49 patients with abnormal cytogenetic findings, sex chromosome abnormalities were observed in 42 (85.71%) including Klinefelter syndrome in 37 (75.5%). Structure chromosome abnormalities involving autosomes (14.28%) and sex chromosomes (2.04%) were detected in 8 infertile men. Furthermore, the Yq microdeletions were seen in two patients (2.22%). Both had complete deletion of the AZFc region.
Conclusion: The occurrence of chromosome anomalies and Yq microdeletions among NOA men strongly suggests genetic testing and counseling prior to employment of assisted reproduction techniques in Tunisia.
Keywords: Male infertility; Non-obstructive azoospermia; Chromosomal abnormalities; Y-chromosome microdeletion
Introduction
Infertility is a major health problem affecting up to 15% of couples of reproductive age [1]. For many years, it was assumed that most reproductive problems could be attributed the female partner, but research in recent years has demonstrated that 30-50% of infertility is caused by male factor [2].
Until recently, there was no treatment available for men who have a complete absence of sperm in the ejaculate (azoospermia). This one accounts 10-15% of male infertility patients [3-5], among them 50-60% of which are non-obstructive azoospermia (NOA), which is characterized by the absence of sperm in the ejaculate without the obstruction of the reproductive tract pathway [6]. NOA occurs in ~1% of the male population and approximately 20-25% of NOA patients are caused by known genetic abnormalities [3, 7, 8]. Those involving chromosome anomalies to about 15%-16% [9]. The most frequent one being the 47, XXY karyotype that characterizes the Klinefelter Syndrome (KS) with a frequency 14% [9], followed by the microdeletions of the long arm of the Y chromosome (Yq) removing the azoospermia factor (AZF) region or parts with a frequency 8% [10]. However, there is still a significant proportion of NOA patients that have unknown etiology.
The establishment of in vitro fertilization using intracytoplasmic sperm injection (ICSI) as a standard treatment modality has resulted in a number of these men successfully fathering a child through surgically retrieved sperm from the testis. However, a genetic risk exists for these offspring, implying the necessity for future parents to be appropriately informed on potential consequences [11-13].
The aim of this study was to determine the prevalence of various chromosomal aberrations and the prevalence of Y-chromosome microdeletions among non-obstructive azoospermia Tunisian men attending the Pasteur Institute of Tunis.
Patients and Methods
Patients
The cytogenetic analysis has been focused on 401 Tunisian infertile patients with idiopathic non-obstructive azoospermia. These infertile men with sperm disorders were referred for karyotyping to the department of Histology and Cytogenetics at the Pasteur Institute of Tunis between January 2006 and May 2014. Among these patients, 90 non-obstructive azoospermia with normal karyotype were benefited from molecular analysis.
Patients were checked for the history of relevant medical disorders, e.g., diabetes, renal, liver disease, radiation, endocrine abnormalities (e.g., hypogonadotropic hypogonadism), exposure to toxins and/or medical affecting spermatogenesis, acquired and congenital structural defect of urogenital system, history of surgical intervention of genital tract.
Informed consent was obtained for karyotype and a molecular investigation from individual participants included in the study, and approval was given by the local ethics committee of Pasteur Institute of Tunis.
Karyotyping
Cytogenetic analysis was performed from phytohemagglutininstimulated lymphocyte cultures by routine laboratory protocol. For microscopic analysis, R-banded metaphase spreads were analyzed and abnormalities recorded according to the current International System for Human Cytogenetic Nomenclature [14]. A resolution of 550 to 700 bands per haploid karyotype was used for the routine analysis. For each patient, at least 20 well-spread metaphases were analyzed and two to five metaphases were karyotyped. When at least one of the 20 showed a loss or gain of a chromosome, especially X or Y chromosome, the number of analyzed metaphases was increased to 30. If a second abnormal cell was observed, the analysis was considered complete; otherwise, the number of metaphases was increased to 50. Sex chromosome mosaics occurring at a level of less 5% were not considered as well as pericentric inversions of chromosome 9 or other structural chromosome variants and polymorphisms that were considered as normal cytogenetic events.
Detection of AZF microdeletions by multiplex PCR
Genomic DNA was extracted from blood lymphocytes using a commercially available kit (FlexiGene Kit; Qiagen), according to the manufacturer’s instructions. Each patient was examined for six AZF loci. The STS primers used were: for AZFa sY84 and sY86, for AZFb sY127 and sY134 and for AZFc sY254 and sY255. This primer set was suggested by Simoni et al. [9] and is prescribed by the European Academy of Andrology (EAA) and European Molecular Genetics Quality Network (EMQN) [15, 16]. In addition, sY14 (STS within the SRY gene located in Yp) was tested as an internal positive control. Two multiplex PCRs were carried out in 50 μl reaction volumes containing: 200 ng of each DNA sample, 1.5mM MgCl2, 0.4 mM of each dNTP, 1.6 μM of each oligonucleotide primer: sY86, sY127, sY254 (Mix A) for the first multiplex PCR, sY84, sY134, sY255 (Mix II) for the second one, 5% dimethyl sulfoxide (DMSO) were added to 1 μl of Taq DNA polymerase. A positive control (sample from a normal fertile male), and two negative controls [(i) normal female sample, (ii) every constituent except DNA], were included in every PCR assay. The reaction mixture included thermo cycling consisted of an initial denaturation of 5 minutes to 94°C followed by 35 cycles of 30 seconds at 94°C, 30 seconds annealing at 59°°C, 30 seconds extension at 720C, finally, 7 minutes extension step at 72°C. PCR products were analyzed by electrophoresis on 3% agarose gels containing ethidium bromide and visualized by exposure to ultraviolet light. In the event of detecting deletion with a primer, the PCR assay was repeated thrice for confirmation. A STS was considered absent only after 3 amplification failures in the presence of successful amplification of internal control (SRY).
Results
Cytogenetic analysis
The average age was 38.07 ± 5.96 and the average duration of infertility was 5.26 ± 4.47 for the azoospermic patients.
Among these 401 patients, 49 cases showed abnormal karyotypes to a prevalence of 12.22%, in which 42 (10.47%) were sex chromosomal abnormalities and 7 (1.75%) were autosomal abnormalities.
The most common abnormality observed was the 47, XXY karyotype or their variant (mosaic 47, XXY/46, XY, 47, XXY/48, XXXY) consistent with KS (Figure 1), which were found in thirty seven cases (9.23%). The other chromosomal abnormalities was represented by: (6) balanced autosomal rearrangements, (2) 46, XX males (Figure 2), (2) unbalanced rearrangements, (1) 47, XYY (Figure 3), and (1) Yq deletion (Figure 4), (Table 1).
% (No. of men with chromosomal aberration/total No.of men karyotyped)
Non-obstructive azoospermia men
12.22 % (49/401)
Sex chromosome aberrations
Autosomal chromosome aberrations
Karyotypes
%
Karyotypes
%
47, XXY
Mosaic 47, XXY:
*46, XY/47, XXY
*47, XXY (85%)/48, XXXY (15%)
47, XYY
45, X (15%)/46, XY (85%)
46, X, del (Y) (q11.2 qter)
XX males (46, XX)
8.23 (33/401)
1 (4/401)
(3)
(1)
0.25 (1/401)
0.25 (1/401)
0.25 (1/401)
0.5 (2/401)
Reciprocal translocation :
*46, XY, t (9 ; 22) (q11;p11)
*46, XY, t (7;16) (p11;p13)
*46, XY, t (4;6) (p12;p22)
*46, XY, t (4;17) (q11;p11)
Robertsonian translocation :
*45, XY, der (13;14) (q10;q10)
Inversion:
*46, XY, inv (7) (q22;q35)
Supernumerary marker chromosomes:
* 46, XY/47, XY, +mar
1 (4/401)
(1)
(1)
(1)
(1)
0.25 (1/401)
(1)
0.25 (1/401)
(1)
0.25 (1/401)
(1)
Subtotal
10.47 (42/401)
Subtotal
1.75 (7/401)
Table 1: Type and frequency of chromosomal anomalies in 401 non-obstructive azoospermia Tunisian men.
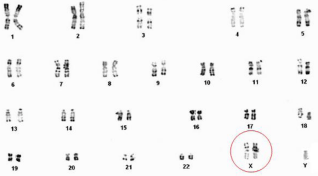
Figure 1: Karyotype of a patient with Klinefelter syndrome: 47, XXY.
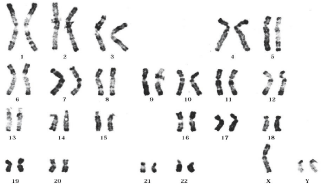
Figure 2: Karyotype of a patient with 47, XYY.
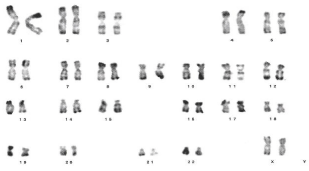
Figure 3: Karyotype of a patient with male syndrome 46, XX.
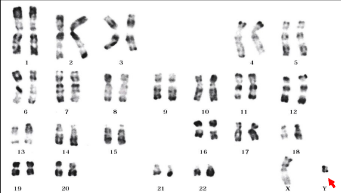
Figure 4: Karyotype of a patient with 46,X,del (Y)(q11.2 qter).
Of balanced rearrangements are identified in six patients including: four with balanced reciprocal translocations, one with balanced Robertsonian translocations between chromosome 13 and chromosome 14 (Figure 9), and one paracentric inversion in chromosome 7 (Figure 10). An unbalanced rearrangement was identified in the two patients: one patient with supernumerary marker chromosome in a low level mosaic [3 out 20 metaphases] (Figure 11), and the last one had three cells with a 45, X constitution.
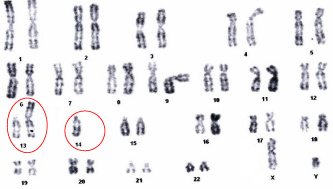
Figure 9: Karyotype of a patient with Robertsonian translocation: 45, XY,
der(13;14)(q10;q10).
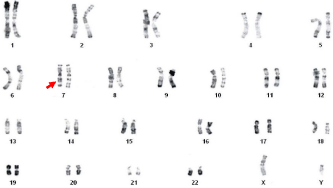
Figure 10: Karyotype of a patient with inversion paracentric of chromosome
7: 46, XY, inv(7)(q22;q35).
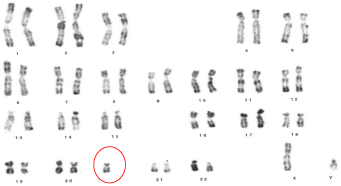
Figure 11: Karyotype of a patient with small supernumerary chromosome:
47, XY, +mar.
Yq chromosome microdeletion screening
Screening for AZF microdeletions was carried out in the 90 NOA men with normal karyotype. Using the EAA/EMQN criteria, AZF region microdeletions on the Y chromosome were found in patients with a frequency of 2.22%. The failed amplification of sY254 and sY255 markers indicates a complete deletion in the AZF sub-region (Figure 12).
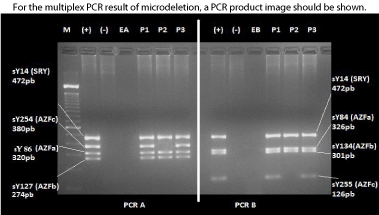
Figure 12: Gel electrophoresis analysis of two multiplex PCRs (PCR A and
B) showing deletion in the AZF region of the Y chromosome.
(P1, P2 & P3) three NOA patient with normal karyotype. (M) size marker
(100bp ladder), (+) fertile man sample, (-) female sample, (EA and EB) water.
Patient (P2) have an absence of the sY254 and sY255 markers, indicating a
complete deletion in the AZFc subregion.
Discussion
Discussion
Chromosomal abnormalities have emerged as one of the major genetic factors contributing to male infertility. In this study, the prevalence of major chromosomal anomalies was 12.22% in NOA with primary infertility. This was lower than in previous studies of Tunisian population 14.10 to 23.62%, but the incidence in other populations has been found to be between 5.43% and 19.44% (Table 2).
Authors
Region
Years
Prevalence of chromosomal aberration (No.of cases /Total No;)
Tuerlings et al. [39]
Netherlands
1998
6.45% (4/62)
Nagvenkar et al. [34]
India
2005
14.29% (6/42)
Elghazel et al.[58]
Tunisia
2006
23.62% (81/343)
Mohammed et al. [59]
Kuwait
2007
19.44% (21/108)
Ng et al. [60]
Hong Kong
2009
21.1% (5/71)
Kosar et al. [61]
South of Turkey
2010
5.43% (5/92)
Mafra et al. [62]
Brazil
2011
11.62% (5/43)
Ghorbel et al. [48]
Tunisia
2012
22.22% (12/54)
Zhang et al. [63]
Northeast China
2012
17.28% (14/81)
Cavkaytar et al. [64]
Turkey
2012
11.22% (22/196)
Al-Achkar et al. [65]
Syria
2013
17.55% (17/97)
Amouri et al. [66]
Tunisia
2014
14.10% (46/328)
This study
Tunisia
2015
12.22% (49/401)
Table 2: Comparison of chromosomal anomalies between this study and other similar studies.
• Sex chromosome abnormalities
Sex chromosome abnormalities are the most frequent chromosome related cause of infertility. In our study, we have found thirty three men (8.23%) with 47, XXY karyotype. A mosaic 46, XY/47, XXY/ 48, XXXY karyotype was found in four azoospermic male (1%). Clinically, these abnormalities are associated with severe spermatogenic failure causing a marked reduction in testicular size which can be associated with gynecomastia, and/or a reduction of pubic and facial and/or hair. And biologically, it is associated with a form of primary hypogonadism. Fluorescence in situ hybridization (FISH) analysis has demonstrated that the frequency of aneuploidy for the sex chromosomes varies from 2% [17] to 45% [18] in the sperm of men who appear to have a non-mosaic KS, and 1.5% [19] to 7% [20] in sperm from mosaics KS. The majority of babies born to men with KS have been normal although chromosomally abnormal fetuses have been reported [21-23] studied embryos by preimplantation genetic diagnosis (PGD) and reported a significant fall in the rate of normal embryos (54%) from KS patients when compared with the controls (72%). Even that there appears to be a small increased risk for these men, it is advised that PGD or prenatal diagnosis be performed before ICSI to ensure that the offspring is not aneuploid [24].
Besides, the second most predominant constituent is represented by the 46, XX, which it has been identified in 2 cases (0.5%). This rare condition was initially named “XX male syndrome”. However, this was revised in October 2005 to its current nomenclature of “46, XX testicular disorder of sex development” (DSD) [25, 26]. This disorder has been reported with an incidence of 0.9% in azoospermic males [27]. Phenotypically the adults are similar to patients with KS. However, 46, XX DSD are shorter, and in some cases they have genital abnormalities [28]. The treatment of fertility of these patients can only be handled using the artificial insemination with donation sperm. Moreover, the donor sperm is banned in Tunisia and in the rest of the Sunni Islamic world [29] and the only possibility paternity for these patients remain the adoption.
The 47, XYY karyotype was seen in one patient. Men with the extra Y chromosome are mostly fertile, but azoospermia may be seen in some cases [30-32].
We also observed in our samples, two others gonosomes aberrations related with the Y chromosome aberration: one case with long arm deletions in all cell, and the second one with the 45X/XY mosaicism constituent. Effectively, loss of genes on the Yq, which plays a primary role in the regulation of different stages of spermatogenesis, is particularly dramatic in spermatozoa production [33].
• Autosomal chromosome abnormalities
The prevalence of autosomal abnormalities in our cohort of NOA was 1.75% (7/401), represented by; six cases with balanced rearrangements [(4) reciprocal translocation, (1) Robertsonian translocation and (1) inversion] and one case with unbalanced rearrangement [(1) small supernumerary marker chromosome (sSMC)].
An association between balanced autosomal translocation and infertility has been reported among NOA men [34-37]. In our samples, the reciprocal translocation were seen four cases involving these translocation [t(9;22); t(4;6); t(7;16); t(4;17)] (Figure 5-8). Otherwise, one case had Robertsonian translocation involving chromosome 13 and 14. Most translocations have no effect on other tissues but can severely impair spermatogenesis [38]. Using the ICSI in this group may increase the inheritance of paternal genetic disorder to offspring due to disrupted meiotic pairing and segregation [38, 39]. Indeed, according to the translocations carried the percentage of unbalanced gametes varies between 2.7% to 26.5% [40]. Both the chromosomes involved in translocation and the location of the breakpoints are likely to be determining factors for the fertility status of the patient. In addition, Robertsonian translocation can result in offspring with Down syndrome or Patau’s syndrome or in gestational loss of concepts with monosomy of chromosome 13, 14 or 21, or trisomy of chromosome 14, which are lethal [32]. Preimplantation genetic diagnosis (PDG) by FISH (Fluorescent in Situ Hybridation) is recommended for the autosomal chromosome aberrations, in order to have an estimation of the risk of abnormal offspring and to adopt genetic counselling which accordingly may be useful for couples who opt for ART.
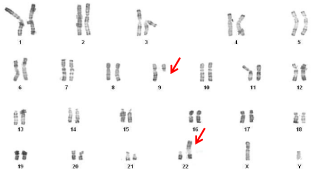
Figure 5: Karyotype of a patient with a balanced reciprocal translocation:
46,XY,t(9;22) (q11; p11).
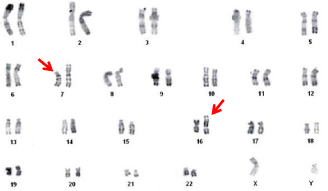
Figure 6: Karyotype of a patient with a balanced reciprocal translocation: 46,
XY, t(7;16) (p11;p13).
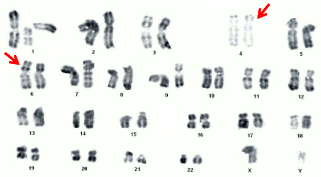
Figure 7: Karyotype of a patient with a balanced reciprocal translocation: 46,
XY, t(4;6)(p12;p22).
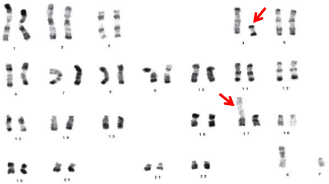
Figure 8: Karyotype of a patient with a balanced reciprocal translocation: 46,
XY, t(4;17)(q11;p11).
A relationship between autosomal inversion and infertility in the male has been reported [41]. The inversions may have complex outcomes, depending on the chromosome and the site and extent of the inversion. Due to the formation of abnormal loops during chromosomal pairing and disruption of meiosis, duplication and deletion events can occur, resulting in a germ cell arrest or the production of sperm with high rates of aneuploidy and consequent adverse birth outcomes [42]. In our study, we have seen only one case with paracentric inversion of chromosome 7. This autosomal abnormality with breakpoint in 7q22 has been reported in other infertile men, from paternal or maternal origin [39, 41]. Furthermore, it is important to document whether structural chromosomal aberration in infertile males are ‘de novo’ or inherited. In case a structural chromosomal aberration is familial and co-segregates with male infertility, this might pinpoint a chromosomal region harboring one or more genes involved in spermatogenesis. Attempts were made to obtain blood samples to karyotype other family members, but in most cases the patients do not concur. Except for the Robertsonian translocation, which was inherited from his normally fertile mother, no further information was obtained for the other cases.
Besides these balanced rearrangements, one case of the 401 NOA men was shown to have small marker supernumerary chromosomes (sSMC). Although the relationship between sSMC and infertile males is not clear, the frequency of sSMC detected in infertile patients is higher than that in general population (0.125% versus 0.043%), and it is also different between male (0.165%) and female infertility (0.022%) [43]. Also, the sSMC with the regions of chromosome 15 or 20 have been reported with male infertility [44, 45], which indicates that male infertility may have no direct relation with genetic abnormality of specific genes. However, sSMC are preferentially maternally transmitted [46], suggesting either a reduced fertility in male carriers or that the marker is excluded in spermatogenesis.
Molecular analysis
The diagnosis of Yq microdeletions is considered to be clinically relevant for appropriate counselling and management of male infertility and is highly recommended for cases undertaking ICSI [16]. The Yq chromosome microdeletions have been investigated in many countries with an overall frequency of 1 to 58%, specifically 8% in NOA men and 3-5% in severely oligozoospermic men [10, 47]. According to the studies carried out by various Tunisian researches, the frequency of AZF microdeletions in NOA varies from 0–54.71% [48, 49]. The large variation in these frequencies could be due to the selection criteria of the patients and partly to molecular testing methodology in relation to the choice and number of STSs used (their position and their reliability).
Using the EAA/EMQN criteria, microdeletions of the Yq were found in two out of the 90 NOA men with normal karyotype (2.22%). The low frequency of the AZF microdeletions in our samples is in accordance with some previous studies reported from Iran (2.13%; 2/94) [50], Algeria (2.04%; 1/49) [51] and Sri Lanka (1.96%; 3/153) [52]. However, this rate is lower than the frequencies reported from Slovenia (3.35%; 8/226) [53], China (9.32%; 33/354) [54], Egypt (39.28%; 11/28) and Brasilia (43.48; 10/23) [55] (Table 3). All these studies were performed by the EAA/EMQN STS markers. The frequency variation among these studies is mainly due to the different factors such as environmental influences and ethnic variation.
Authors
Region
Years
Prevalence of Yq microdeletions (No. of cases /Total)
Karyotype
Arruda et al. [55]
Brasilia
2007
43.48% (10/23)
46, XY
Elhawary et al.[67]
Egypt
2010
39.28% (11/28)
46, XY
Behulova et al.[53]
Slovakia
2011
3.35% (8/226)
46, XY
Sun et al. [54]
China (Shanghai)
2012
9.32% (33/354)
46, XY
Wettasinghe et al. [52]
Sri Lanka
2012
1.96% (3/153)
46, XY
Saliminejad et al.[50]
Iran
2012
2.13% (2/94)
46, XY
Chellat et al. [51]
Alegria
2013
2.04% (2/49)
NA
Hammami et al. [68]
Tunisia
2014
2.70% (2/74)
46, XY
This study
Tunisia
2015
2.22% (2/90)
46, XY
NA= not available;
Table 3: Frequency of Yq microdeletions in non-obstructive azoospermic men with normal karyotype screened with the EAA/EMQN STSs markers from different region.
The two NOA with the Yq microdeletions had a complete microdeletion of the AZFc region (failed amplification of sY254 and sY255 markers). None of them in the present study, showed deletion neither in AZFa nor in AZFb regions showed Yq microdeletions. Microdeletions in the AZFa and AZFb regions are extremely rare while AZFc microdeletion is the most frequently detected in the most published reviews [15]. Histologically, the AZFc microdeletion is associated with various spermatogenetic alterations. Furthermore, AZFc deletions phenotype is less severe because, these deletions are compatible with residual spermatogenesis and, in rare cases, can even be transmitted naturally to the male offspring [56, 57].
Conclusion
Although there is still a significant proportion of NOA patients that have unknown etiology, the cytogenetic and the molecular exploration still has an important role in the workup of male infertility of viewpoint diagnostic, prognostic and preventive in couples who wants to undergo ICSI treatment. Once a genetic cause is observed in an infertile man it helps the clinicians to avoid empirical and often expensive treatments to improve fertility.
Acknowledgment
We are thankful to the patients for their collaboration in the present study and the excellent technical assistance provided by Miss Imen Chemkhi, Miss Nabila Abidli, Mr. Helmi Guermani, and Mr. Sofien Hentati are gratefully acknowledged.
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR11IPT05), the European commission funded FP7 project GM_NCD_ INCO (GENOMEDIKA), the Tunisian Ministry of Public Health and the Project to Support Research and Innovation System (PASRI) MOBIDOC.
References
- Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996; 11 Suppl 4: 1-24.
- Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility--a systematic review of prevalence studies. Hum Reprod Update. 2011; 17: 575-588.
- Maduro MR, Lamb DJ. Understanding new genetics of male infertility. J Urol. 2002; 168: 2197-2205.
- Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. J Urol. 2002; 167: 2138-2144.
- Jarvi K, Lo K, Fischer A, Grantmyre J, Zini A, Chow V, et al. CUA Guideline: The workup of azoospermic males. Can Urol Assoc J. 2010; 4: 163-167.
- Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo). 2013; 68 Suppl 1: 27-34.
- Tu W, Liu Y, Shen Y, Yan Y, Wang X, Yang D, et al. Genome-wide Loci linked to non-obstructive azoospermia susceptibility may be independent of reduced sperm production in males with normozoospermia. Biol Reprod. 2015; 92: 41.
- Lee JY, Dada R, Sabanegh E, Carpi A, Agarwal A. Role of genetics in azoospermia. Urology. 2011; 77: 598-601.
- Krausz C, Chianese C. Genetic testing and counselling for male infertility. Curr Opin Endocrinol Diabetes Obes. 2014; 21: 244-250.
- Simoni M, Tüttelmann F, Gromoll J, Nieschlag E. Clinical consequences of microdeletions of the Y chromosome: the extended Münster experience. Reprod Biomed Online. 2008; 16: 289-303.
- Coat C, Perrin A, Talagas M, Tetefort R, Amice J, Valéri A, et al. [Azoospermia: management and results: a series of 90 cases]. Prog Urol. 2011; 21: 946-954.
- Oldereid NB, Hanevik HI, Bakkevig I, Romundstad LB, Magnus O, Hazekamp J, et al. Pregnancy outcome according to male diagnosis after ICSI with non-ejaculated sperm compared with ejaculated sperm controls. Reprod Biomed Online. 2014; 29: 417-423.
- Kumar R. Medical management of non-obstructive azoospermia. Clinics (Sao Paulo). 2013; 68 Suppl 1: 75-79.
- Shaffer L, Slovak M, Campbell L: International Standing Committee on Human Cytogenetic Nomenclature. ISCN 2009: An International System for Human Cytogenetic Nomenclature 2009.
- Krausz C, Hoefsloot L, Simoni M, Tüttelmann F. European Academy of Andrology; European Molecular Genetics Quality Network. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state-of-the-art 2013. Andrology. 2014; 2: 5-19.
- Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions. State of the art 2004. Int J Androl. 2004; 27: 240-249.
- Rives N, Joly G, Machy A, Siméon N, Leclerc P, Macé B. Assessment of sex chromosome aneuploidy in sperm nuclei from 47,XXY and 46,XY/47,XXY males: comparison with fertile and infertile males with normal karyotype. Mol Hum Reprod. 2000; 6: 107-112.
- Estop AM, Munné S, Cieply KM, Vandermark KK, Lamb AN, Fisch H. Meiotic products of a Klinefelter 47,XXY male as determined by sperm fluorescence in-situ hybridization analysis. Hum Reprod. 1998; 13: 124-127.
- Lim AS, Fong Y, Yu SL. Estimates of sperm sex chromosome disomy and diploidy rates in a 47,XXY/46,XY mosaic Klinefelter patient. Hum Genet. 1999; 104: 405-409.
- Kruse R, Guttenbach M, Schartmann B, Schubert R, van der Ven H, Schmid M. Propping P: Genetic counseling in a patient with XXY/XXXY/XY mosaic Klinefelter's syndrome: estimate of sex chromosome aberrations in sperm before intracytoplasmic sperm injection. Fertil Steril. 1998; 69: 482-485.
- Ron-El R, Strassburger D, Gelman-Kohan S, Friedler S, Raziel A, Appelman Z. A 47, XXY fetus conceived after ICSI of spermatozoa from a patient with non-mosaic Klinefelter's syndrome: case report. Hum Reprod. 2000; 15: 1804-1806.
- Friedler S, Raziel A, Strassburger D, Schachter M, Bern O, Ron-El R. Outcome of ICSI using fresh and cryopreserved-thawed testicular spermatozoa in patients with non-mosaic Klinefelter's syndrome. Hum Reprod. 2001; 16: 2616-2620.
- Staessen C, Tournaye H, Van Assche E, Michiels A, Van Landuyt L, Devroey P, et al. PGD in 47, XXY Klinefelter's syndrome patients. Hum Reprod Update. 2003; 9: 319-330.
- Georgiou I, Syrrou M, Pardalidis N, Karakitsios K, Mantzavinos T, Giotitsas N, et al. Genetic and epigenetic risks of intracytoplasmic sperm injection method. Asian J Androl. 2006; 8: 643-673.
- Hughes IA, Houk C, Ahmed SF, Lee PA. Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology Consensus Group. Consensus statement on management of intersex disorders. J Pediatr Urol. 2006; 2: 148-162.
- Lee PA, Houk CP, Ahmed SF, Hughes IA. International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology . Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006; 118: e488-500.
- Mau-Holzmann UA. Somatic chromosomal abnormalities in infertile men and women. Cytogenet Genome Res. 2005; 111: 317-336.
- Boucekkine C, Toublanc JE, Abbas N, Chaabouni S, Ouahid S, Semrouni M, et al. Clinical and anatomical spectrum in XX sex reversed patients. Relationship to the presence of Y specific DNA-sequences. Clin Endocrinol (Oxf). 1994; 40: 733-742.
- Inhorn MC, Patrizio P, Serour GI. Third-party reproductive assistance around the Mediterranean: comparing Sunni Egypt, Catholic Italy and multisectarian Lebanon. Reprod Biomed Online. 2010; 21: 848-853.
- El-Dahtory F, Elsheikha HM. Male infertility related to an aberrant karyotype, 47, XYY: four case reports. Cases J. 2009; 2: 28.
- Sadeghi-Nejad H, Farrokhi F. Genetics of azoospermia: current knowledge, clinical implications, and future directions. Part I. Urol J. 2006; 3: 193-203.
- Martin RH. Cytogenetic determinants of male fertility. Hum Reprod Update. 2008; 14: 379-390.
- Valetto A, Bertini V, Rapalini E, Baldinotti F, Di Martino D, Simi P. Molecular and cytogenetic characterization of a structural rearrangement of the Y chromosome in an azoospermic man. Fertil Steril. 2004; 81: 1388-1390.
- Nagvenkar P, Desai K, Hinduja I, Zaveri K. Chromosomal studies in infertile men with oligozoospermia & non-obstructive azoospermia. Indian J Med Res. 2005; 122: 34-42.
- Pandiyan N, Jequier AM. Mitotic chromosomal anomalies among 1210 infertile men. Hum Reprod. 1996; 11: 2604-2608.
- Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology. 2014; 2: 339-350.
- Dong Y, Du RC, Jiang YT, Wu J, Li LL, Liu RZ . Impact of chromosomal translocations on male infertility, semen quality, testicular volume and reproductive hormone levels. J Int Med Res. 2012; 40: 2274-2283.
- Dada R, Thilagavathi J, Venkatesh S, Esteves SC, Agarwal A. Genetic testing in male infertility. Open Reprod Sci J. 2011; 3: 42-56.
- Tuerlings J, de France HF, Hamers A, Hordijk R, Van Hemel JO, Hansson K, et al. Chromosome studies in 1792 males prior to intra-cytoplasmic sperm injection: the Dutch experience. Eur J Hum Genet. 1998; 6: 194-200.
- Moradkhani K, Puechberty J, Bhatt S, Lespinasse J, Vago P, Lefort G, et al. Rare Robertsonian translocations and meiotic behaviour: sperm FISH analysis of t(13;15) and t(14;15) translocations: a case report. Hum Reprod. 2006; 21: 3193-3198.
- Faed MJ, Robertson J, Lamont MA, MacIntosh W, Grieve J, Baxby K, James GB . A cytogenetic survey of men being investigated for subfertility. J Reprod Fertil. 1979; 56: 209-216.
- McLachlan RI, O'Bryan MK. Clinical Review#: State of the art for genetic testing of infertile men. J Clin Endocrinol Metab. 2010; 95: 1013-1024.
- Liehr T. Weise A: Frequency of small supernumerary marker chromosomes in prenatal, newborn, developmentally retarded and infertility diagnostics. Int J Mol Med. 2007; 19: 719-731.
- Eggermann K, Mau UA, Bujdosó G, Koltai E, Engels H, Schubert R, et al. Supernumerary marker chromosomes derived from chromosome 15: analysis of 32 new cases. Clin Genet. 2002; 62: 89-93.
- McNerlan SE, Morrison PJ, McClure N, Nevin NC. A supernumerary chromosome 20, identified by FISH, in a male with azoospermia-cause or coincidence? Am J Med Genet A. 2003; 117A: 188-190.
- Buckton KE, Spowart G, Newton MS, Evans HJ. Forty four probands with an additional "marker" chromosome. Hum Genet. 1985; 69: 353-370.
- Suganthi R, Vijesh VV, Vandana N, Fathima Ali Benazir J. Y choromosomal microdeletion screening in the workup of male infertility and its current status in India. Int J Fertil Steril. 2014; 7: 253-266.
- Ghorbel M, Gargouri Baklouti S, Ben Abdallah F, Zribi N, Cherif M, Keskes R, et al. Chromosomal defects in infertile men with poor semen quality. J Assist Reprod Genet. 2012; 29: 451-456.
- Hadjkacem-Loukil L, Ayadi I, Bahloul A, Ayadi H, Ammar-Keskes L. Tag STS in the AZF region associated with azoospermia in a Tunisian population. J Androl. 2007; 28: 652-658.
- Saliminejad K, Sadeghi MR, Kamali K, Amirjannati N, Soltanghoraee H, Khorram Khorshid HR. Discrepancy in the frequency of Y chromosome microdeletions among Iranian infertile men with azoospermia and severe oligozoospermia. Genet Test Mol Biomarkers. 2012; 16: 931-934.
- Chellat D, Rezgoune ML, McElreavey K, Kherouatou N, Benbouhadja S, Douadi H, et al. First study of microdeletions in the Y chromosome of Algerian infertile men with idiopathic oligo- or azoospermia. Urol Int. 2013; 90: 455-459.
- Wettasinghe TK, Jayasekara RW, Dissanayake VH. The low frequency of Y chromosome microdeletions in subfertile males in a Sinhalese population of Sri Lanka. Indian J Hum Genet. 2012; 18: 320-325.
- Behulova R, Varga I, Strhakova L, Bozikova A, Gabrikova D, Boronova I, et al. Incidence of microdeletions in the AZF region of the Y chromosome in Slovak patients with azoospermia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011; 155: 33-38.
- Sun K, Chen XF, Zhu XB, Hu HL, Zhang W, Shao FM, et al. A new molecular diagnostic approach to assess Y chromosome microdeletions in infertile men. J Int Med Res. 2012; 40: 237-248.
- Arruda JT, Bordin BM, Santos PR, Mesquita WE, Silva RC, Maia MC, et al. Y chromosome microdeletions in Brazilian fertility clinic patients. Genet Mol Res. 2007; 6: 461-469.
- Patsalis PC, Sismani C, Quintana-Murci L, Taleb-Bekkouche F, Krausz C, McElreavey K. Effects of transmission of Y chromosome AZFc deletions. Lancet. 2002; 360: 1222-1224.
- Oates RD, Silber S, Brown LG, Page DC. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod. 2002; 17: 2813-2824.
- Elghezal H, Hidar S, Braham R, Denguezli W, Ajina M, Saâd A. Chromosome abnormalities in one thousand infertile males with nonobstructive sperm disorders. Fertil Steril. 2006; 86: 1792-1795.
- Mohammed F, Al-Yatama F, Al-Bader M, Tayel SM, Gouda S, Naguib KK. Primary male infertility in Kuwait: a cytogenetic and molecular study of 289 infertile Kuwaiti patients. Andrologia. 2007; 39: 87-92.
- Ng PP, Tang MH, Lau ET, Ng LK, Ng EH, Tam PC, et al. Chromosomal anomalies and Y-microdeletions among Chinese subfertile men in Hong Kong. Hong Kong Med J. 2009; 15: 31-38.
- Koşar PA, Ozçelik N, Koşar A. Cytogenetic abnormalities detected in patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet. 2010; 27: 17-21.
- Mafra FA, Christofolini DM, Bianco B, Gava MM, Glina S, Belangero SI, et al. Chromosomal and molecular abnormalities in a group of Brazilian infertile men with severe oligozoospermia or non-obstructive azoospermia attending an infertility service. Int Braz J Urol. 2011; 37: 244-250.
- Zhang ZB, Jiang YT, Yun X, Yang X, Wang RX, Dai RL, et al. Male infertility in Northeast China: a cytogenetic study of 135 patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet. 2012; 29: 83-87.
- Cavkaytar S, Batioglu S, Gunel M, Ceylaner S, Karaer A. Genetic evaluation of severe male factor infertility in Turkey: a cross-sectional study. Hum Fertil (Camb). 2012; 15: 100-106.
- Al-Achkar W, Wafa A, Moassass F. Cytogenetic abnormalities and Y-chromosome microdeletions in infertile Syrian males. Biomed Rep. 2013; 1: 275-279.
- Amouri A, Hammami W, Kilani O, Bouzouita A, Ayed W, Ben Meftah M, Khrouf M, et al. Chromosomal evaluation in a group of Tunisian patients with non-obstructive azoospermia and severe oligozoospermia attending a Tunisian cytogenetic department. C R Biol. 2014; 337: 223-228.
- Elhawary NA, Seif-Eldin NS, Zaki M, Diab H, Teama S, Saleh SA. Common Tag STSs in the AZF Region Associated with Azoospermia and Severe Oligospermia in Infertile Egyptian Men. Open Androl J. 2010; 2: 11-18.
- Hammami W, Kilani O, Ben Khelifa M, Ayed W, Abdelhak S, Bouzouita A, et al. Prevalence of Y chromosome microdeletions in infertile Tunisian men. Ann Biol Clin (Paris). 2014; 72: 331-336.