
Research Article
Austin J Reprod Med Infertil. 2015;2(3): 1014.
A Comparison of Sperm DNA Damage in the Neat Ejaculate of Sperm Donors and Males Presenting for their Initial Seminogram
Jaime Gosálvez¹*, José-LuisFernández², Jesus Yaniz³, Moises de la Casa4, Carmen López-Fernández1 and Stephen Johnston5
1Unit of Genetics, Department of Biology, Universidad Autónoma de Madrid, 20849 Madrid, Spain
2GeneticsUnit, Complejo Hospitalario Universitario A Coruña-INIBIC, A Coruña, Spain
3TECNOGAM, Instituto Universitario de Ciencias Ambientales (IUCA) and Departamento de Producción Animal y Ciencia de los Alimentos, University of Zaragoza, Spain
4GINEFIV, Calle José Silva, 18, 28043 Madrid, Spain
5School of Agriculture and Food Science, The University of Queensland, Australia
*Corresponding author: Jaime Gosálvez, Unit of Genetics, Department of Biology, Universidad Autónoma de Madrid, Spain
Received: April 14, 2015; Accepted: June 24, 2015; Published: June 29, 2015
Abstract
A standardised value for characterization of sperm DNA fragmentation (SDF) and fertility prediction in patients is regarded as controversial and is one of the primary reasons why the assessment of the sperm DNA is not commonly incorporated into the routine seminogram. To address this conundrum we have conducted an analysis of the incidence of sperm DNA damage between sperm donors and a random cohort of males requested for a first seminogram. The underlying assumption was that sperm DNA damage is a neutral sperm characteristic and differences would not be expected between both cohorts. We analyzed SDF using the sperm chromatin dispersion assay in two large cohorts of individuals that included a comparison of seminograms from 210 sperm donors and 775 couples presenting to the clinic for first time. Results of the analysis revealed that sperm DNA damage cannot be considered as neutral parameter between the two populations (Donor: SDF Mean = 10.7; SD: 8.7; First seminogram: Mean =29.2; SD: 17.6; U-Mann-Whitney 20486; P 0.000). Receiving operating characteristics curves constructed under the parameters of this study showed that a SDF value of 16% was able to discriminate both cohorts of individuals with a sensitivity of 85% and a specificity of 75%. We suggest that when clinics are selecting for sperm donors, they are also likely to be indirectly selecting for a level of SDF which is in the order of 20 points lower than the level found in those couples presenting to the clinic for the first time.
Keywords: Andrology; Sperm DNA fragmentation; Male factor; Sperm donors
Introduction
Even after 30 years of clinical practice, urologists have yet to reach general consensus about the precise role of sperm DNA fragmentation (SDF) in male infertility [1,2]. A lack of precision when using this parameter to predict fertility is no doubt contributing to this sense of controversy and preventing the universal inclusion of SDF as part of the standard seminogram; we propose a range of reasons for this variability. The first of these issues has to do with the plethora of possible pathologies associated with the original aetiology of sperm DNA damage. Sperm DNA damage has been linked to oxidative stress, protamination failure, meiotic failure and/or abortive apoptosis [1- 7]. Secondly, in addition to these organic causes, elevated levels of SDF have also been linked to lifestyle, pollution, bacterial infections and even high frequency energy exposure [8-10]. Thirdly, we must also be conscious of so-called “iatrogenic sperm DNA damage, which may be caused inadvertently as a result of improper ex vivo sperm handling or manipulation [11,12]. Finally, it is possible that the lack of predictability could also be associated with respect to the how and when the DNA damage is measured and what specific sperm delivery protocols are used to fertilise the oocyte or to prepare the sperm prior to syngamy. It is not surprising therefore, to find different threshold levels of sperm DNA fragmentation based on the specific fertilization procedures employed, for example, compare intrauterine insemination with ICSI [13].
Given the controversy that abounds about the actual role of SDF in fertility and for the purposes of this study, we have made the assumption, that SDF is a neutral sperm characteristic, i.e. it is an irrelevant sperm characteristic that may equally fluctuate irrespectively on the male that is analysed. For this null hypothesis to be upheld, we would expect to see no major difference in its prevalence when two different cohorts of individuals are compared. Consequently, we shall compare the level of SDF in the ejaculates of a sperm donor population with that of a large population of males presenting to the clinic for their first seminogram.
Material and Methods
This was a blind, retrospective, large cohort population study to assess the prevalence of SDF in sperm of donors (Group D; n=210) and males of couples presenting to a reproductive technology clinic for a Fist Seminogram: (Group FS; n=775). The study was conducted under the informed consent of all participants and according to the guidelines and protocols of the clinic’s ethical committee under the research project BFU-2013-44990R. The age range for Group D and FS was 23 ± 2.1 and 33 ± 12.3 years, respectively. Donors were selected using standard criteria where no personal or family chronic or serious illness that can affect offspring and no history of birth defects in family history, were considered. Negative serology for HIV, hepatitis B and C, syphilis, cytomegalovirus, and no trace of diseases such as Chlamydia and Gonococcus were also included. Normal karyotype and normo-zoospermia for seminogram before and after sperm cryopreservation were also considered as criteria for inclusion in the study.
SDF was directly assessed from the neat ejaculate immediately following sample liquefaction. This approach negated the adverse impact of iatrogenic damage as a confounding factor of the analysis [11]. SDF was assessed using Halosperm (Halotech DNA, Madrid, Spain). Details of the sperm chromatin dispersion test as used in the present investigation have been described elsewhere in previous studies [14]. Statistical analyses were performed with the Statistical Package for Social Sciences (SPSS v.11; Chicago, IL, USA). The test for normal data distribution was performed using Z-Kolmogorov- Smirnov and data comparison was conducted using a non-parametric Mann-Whitney U test, using anα of 0.05. Receiving operating characteristic (ROC curves) to assess for sensitivity and specificity were run under consideration of non-parametric analysis.
Results
Descriptive statistics
The frequency distribution of the different subclasses of SDF following grouping of the data in terms of increasing and rank-size SDF values is shown in Figure 1. Sperm DNA fragmentation values did not conform to a normal distribution (Z-Kolmogorov-Smirnov for Group D = 2.52; P 0.000 / Z-Kolmogorov-Smirnov for Group P = 3.461; P 0.000; Figure 1); there was a common tendency for both groups to displace the curve towards a greater incidence of low values for SDF. Descriptive statistics for both groups are shown in Figure 2a; Group FS (Mean = 29.2; Median: 26.2; SD: 17.6) had a higher SDF value than group D (Mean = 10.7; Median: 9.0; SD: 8.7) (U-Mann- Whitney 20486; P 0.000).
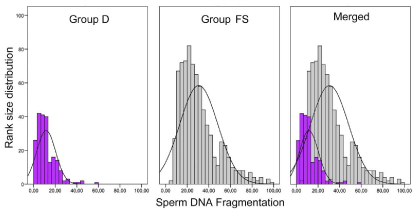
Figure 1: Class histogram and associated curve showing the distribution of
sperm DNA fragmentation in Group D (Donors), Group FS (First Seminogram)
and merged data.
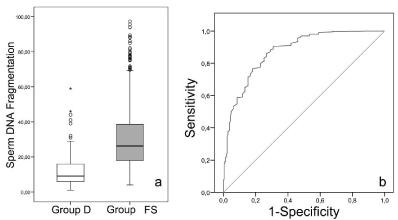
Figure 2: (a) Box-whisker diagrams summarizing descriptive statistics for
Group D (Donors) and Group FS (First Seminogram); (b) Receiver operating
characteristic curve using Group D as discriminative characteristic.
ROC curve analysis
Given the large difference in mean SDF between the two groups, we tested the sensitivity and specificity to determine whether it was possible to discriminate between Group D and Group FS by calculating the area under the ROC curve. Using this statistical approach SDF assessment resulted in a significant area under the curve (AUC) for predicting the inclusion of an individual in Group D. The AUC value obtained was 0.874 (P 0.000, based on non-parametric data assumption; Figure 2b). The Youden’s J statistic, a single statistic that captures the performance of a diagnostic test, showed that using a cutoff value of 16% for SDF, it was possible to predict that that and individual can be included within Group D with a sensitivity of 85% and a specificity of 75% (Figure 2b).
Discussion
The results obtained in the present investigation have clearly shown that SDF cannot be considered a neutral semen characteristic. When a highly selected large population of semen donors was compared with an even larger population of randomized putative patients undergoing their first seminogram; the FS cohort had a mean SDF value 20 points higher than donor semen samples. From this finding, we would suggest that individuals included in Group D and presenting with a low level of SDF fragmentation not exceeding a SDF value of 16% could be considered as the reference standard of a sperm donor. The ROC curve analysis indicated this value is predictive with a sensitivity and specificity of 85% and 75%, respectively. The threshold values obtained for this study are, of course, only relevant for the conditions under which the present study was conducted whereby (1) all the semen samples were freshly collected, (2) all semen samples were exposed to the same degree of iatrogenic damage, (3) the SDF procedure was conducted on neat semen samples and (4) the SDF was assessed using a sperm chromatin dispersion test. It is interesting to note that both data sets did not conform to a normal distribution and there was a tendency for both groups to be skewed towards low values of SDF. This could be interpreted as a natural tendency in the human population to generally exhibit low levels of sperm DNA damage in the ejaculate; a fact that was notably reinforced in the donor population. Although not an objective of the present study, we would expect that the valuesfor SDF should be lower after sperm selection procedures using classic strategies such as “swim up” or density gradient centrifugation for sperm selection. These methodologies tend to diminish the level of SDF observed in the neat ejaculate [15-17].
In relation to fertility, the benefit of using selected spermatozoa to improve pregnancy rate has been known for a long time [18]. This is logical for when using ICSI the probability of selecting a sperm free of DNA damage should increase if density gradient centrifugation or swim-up procedures are employed. Congruent with this idea, some reports show that the predictive value of SDF tends to be low when ICSI is used [13, 19] and this is mainly because part of semen sample containing damaged DNA has been removed from the original neat ejaculate. This reduction in SDF which is linked to a positive effect on pregnancy after ICSI can also be considered as non-direct evidence of the importance of SDF at the time of reproductive outcome.
SDF values associated with predicting pregnancy and threshold values are a controversial issue and studies where SDF are assessed specifically on donor populations are rare [20]. Recently, Belloc et al. [21] have shown that SDF in normozoospermic infertile men was negatively correlated with sperm motility but positively correlated with the age of the patient; in this study, 11% of the patients exhibited unusually high levels of sperm DNA fragmentation. In our case, we have found that 15% of individuals within group FS showed abnormal SDF values higher than 50%, while these values declined to less than 0.5 % in group D. SDF values of higher than 30%, which is one of the threshold and critical values correlated with fertility [22], only affected less than 4% of the donors. These results provide evidence to support the observation that assisted reproductive clinics are potentially unknowingly selecting for low levels of SDF in the donor population; this is an interesting idea that requires further analysis. Nonetheless, in other mammals, where artificial selection is regularly performed to increase reproductive capacity, the incidence of SDF is also low. In fact, SDF in selected breeders rarely surpasses 5% [23]. In this case, artificial insemination centers do not normally identify animals with a high or low level of SDF, simply because is not a common parameter assessed on a routine basis. It would therefore seem that such artificial selection is a relatively blind form of genetic engineering with little knowledge of the genes they are selecting for. For example, in boar the effects of selection for increased size of testes on standard semen characteristics have been analyzed and differences in sperm characteristics and sperm production were consistent with the and increase in the size of the testicles. We would predict that this type of selection is also concomitant with a decrease in the levels of DNA damage, mainly because we are blindly selecting for other genes or traits linked to the production of good sperm quality. Thus, sperm DNA packing and/or protamination characteristics, the capacity to control oxidative stress [24,25] and mechanisms depending on the triggering of apoptotic-like related processes associated with spermatogenic failure [26,27], some of them having a genetic background, are all indirectly and positively selected when we control and choice, for breeding purposes, animals presenting a high quality in standard seminal characterisitics.
The next question to be asked from this line of argument might be“Are donor individuals with SDF values of lower than 16% more efficient for reproductive outcome than those greater than this value?” In other words, is a SDF value of lower than 16%, a characteristic of outstanding sperm quality?
Acknowledgement
Financial support for this study was provided a Governmental Research Grant (BFU2013-44290-R). The authors report no conflict of interest.
References
- Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980; 210: 1131-1133.
- Drobnis EZ, Johnson MH. Are we ready to incorporate sperm DNA-fragmentation testing into our male infertility work-up? A plea for more robust studies. Reprod Biomed Online. 2015; 30: 111-112.
- Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999; 4: 31-37.
- Aitken J, Krausz C, Buckingham D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994; 39: 268-279.
- Ko EY, Sabanegh ES Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014; 102: 1518-1527.
- Sakkas D, Manicardi G, Bianchi PG, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biol Reprod. 1995; 52: 1149-1155.
- Moskovtsev SI, Willis J, White J, Mullen JB. Sperm DNA damage: correlation to severity of semen abnormalities. Urology. 2009; 74: 789-793.
- Kumar S, Murarka S1, Mishra VV, Gautam AK. Environmental & lifestyle factors in deterioration of male reproductive health. Indian J Med Res. 2014; 140 Suppl: S29-35.
- Gallegos G, Ramos B, Santiso R, Goyanes V, Gosálvez J, Fernández JL. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil Steril. 2008; 90: 328-334.
- De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. 2009; 4: e6446.
- Gosálvez J, López-Fernández C, Fernández JL, Gouraud A, Holt WV. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol Reprod Dev. 2011; 78: 951-961.
- O'Flaherty C. Iatrogenic genetic damage of spermatozoa. Adv Exp Med Biol. 2014; 791: 117-135.
- Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004; 19: 1401-1408.
- Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005; 84: 833-842.
- Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000; 56: 1081-1084.
- Enciso M, Iglesias M, Galán I, Sarasa J, Gosálvez A, Gosálvez J. The ability of sperm selection techniques to remove single- or double-strand DNA damage. Asian J Androl. 2011; 13: 764-768.
- Gosálvez J, Caballero P, López-Fernández C, Ortega L, Guijarro JA, Fernández JL, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013; 15: 812-818.
- Tanphaichitr N, Agulnick A, Seibel M, Taymor M. Comparison of the in vitro fertilization rate by human sperm capacitated by multiple-tube swim-up and Percoll gradient centrifugation. J In Vitro Fert Embryo Transf. 1988; 5: 119-122.
- Castilla JA, Zamora S, Gonzalvo MC, Luna Del Castillo JD, Roldan-Nofuentes JA, Clavero A, et al. Sperm chromatin structure assay and classical semen parameters: systematic review. Reprod Biomed Online. 2010; 20: 114-124.
- Gosálvez J, Cortés-Gutierez E, López-Fernández C, Fernández JL, Caballero P, Nuñez R. Sperm deoxyribonucleic acid fragmentation dynamics in fertile donors. Fertil Steril. 2009; 92: 170-173.
- Belloc S, Benkhalifa M, Cohen-Bacrie M, Dalleac A, Amar E, Zini A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil Steril. 2014; 101: 1588-1593.
- Evenson DP, Wixon R. Data analysis of two in vivo fertility studies using Sperm Chromatin Structure Assay-derived DNA fragmentation index vs pregnancy outcome. FertilSteril. 2008; 90: 1229–1231.
- Gosálvez J, William V. Holt WV, Johnston DS. Sperm DNA fragmentation and its role in wildlife conservation.Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology (Springer, New York). 2014; 753: 357-384.
- Ji G, Yan L, Liu W, Qu J, Gu A. OGG1 Ser326Cys polymorphism interacts with cigarette smoking to increase oxidative DNA damage in human sperm and the risk of male infertility. Toxicol Lett. 2013; 218: 144-149.
- Chengyong W, Man Y, Mei L, Liping L, Xuezhen W. GSTM1 null genotype contributes to increased risk of male infertility: a meta-analysis. J Assist Reprod Genet. 2012; 29: 837-845.
- Ricci G, Perticarari S, Fragonas E, Giolo E, Canova S, Pozzobon C, et al. Apoptosis in human sperm: its correlation with semen quality and the presence of leukocytes. Hum Reprod. 2002; 17: 2665-2672.
- Wang X, Sharma RK, Sikka SC, Thomas AJ Jr, Falcone T, Agarwal A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003; 80: 531-535.