
Research Article
Austin J Reprod Med Infertil. 2015;2(3): 1015.
Fresh Blastocyst Embryo Transfer is Superior to Morula Embryo Transfer in Young Patients Undergoing in Vitro Fertilization
Malik S¹, Balassiano E¹, Hobeika E¹, Knochenhauer ES¹,² and Traub ML¹,²*
1Department of Obstetrics and Gynecology, Staten Island University Hospital, USA, 475 Seaview Avenue, Staten Island, NY, USA
2Island Reproductive Services, USA, 1110 South Avenue,Suite 305, Staten Island, NY, USA
*Corresponding author: Traub ML, Department of Obstetrics and Gynecology, Staten Island University Hospital, Island Reproductive Services, USA
Received: April 16, 2015; Accepted: June 29, 2015; Published: June 30, 2015
Abstract
Objective: To determine if blastocyst embryo transfer yields better pregnancy outcomes compared to morula embryo transfer for fresh and frozen cycles and in donor oocyte recipients.
Study Design: Retrospective cohort of patients undergoing in vitro fertilization at a single center. Fresh, frozen, and donor egg recipient cycles between January 1, 2008 and December 31, 2012 were studied. Patients were excluded if they were considered poor prognosis and underwent day 3 embryo transfers.
Results: In patients under age 35 undergoing fresh IVF cycle, implantation rates (52% v 29%, p<0.01), clinical pregnancy rates (63% v 38%, p=0.001), and live birth rates (54% v 33%, p<0.01) were all higher after blastocyst embryo transfer. No differences were seen in other SART age groups during fresh IVF. For patients undergoing FET and in donor oocyte recipients, no differences in any pregnancy outcome were between blastocyst and morula embryo transfer.
Conclusions: Blastocyst embryo transfer was found to improve pregnancy outcomes in young patients undergoing fresh IVF. The data provides the first studies of morula in FET and donor cycles and found no benefit in these populations to blastocyst embryo transfer. Our study confirmed good implantation rates after morula embryo transfer.
Keywords: Embryo transfer; In vitro fertilization; Morula; Blastocyst; Infertility
Abbreviations
IVF: In Vitro Fertilization; FET: Frozen Embryo Transfer; FSH: Follicle Stimulating Hormone
Introduction
Blastocystembryo transfer is believed to improve pregnancy rates with in vitro fertilization (IVF) compared to cleavage stage embryos transfer as a result of better embryo selection, genomic activation, better embryo-uterine synchrony, and lower estradiol levels [1,2]. Blastocyst transfer may increase the incidence of monozygotic twinning [3-5] and imprinting disorders [6,7] compared to cleavage stage embryos. Morula-stage embryos share a similar advantage to blastocyst-stage embryos because their genome is activated. Morula (day 4) transfers are not typically performed since morphological assessment is more difficult – blastomeresare less distinct and differentiation of the inner cell mass and trophectoderm are not yet possible [8]. No studies have compared differences in imprinting and monozygotic twinning between morula and blastocyst stage embryos [9].
Morula embryo transfer was first reported in the literature in 1998 for poor prognosis patients undergoing pre implantation genetic diagnosis after blastomere biopsy [10,11]. Comparisons between fresh cleavage stage and morula embryo transfers showed similar clinical pregnancy rates [8,12] with other studies confirming reasonable implantation and live birth rates [13,14]. The few studies which have compared fresh morula to blastocyst embryo transfer showed equivalent clinical pregnancy rates with limited live birth data, and the cycles all took place prior to 2009 [15,16]. A recent retrospective study of fresh cycles between 2006 and 2009 showed lower implantation rates with morula transfer but did not report live birth data [17]. No publications to date have compared frozen embryo transfer (FET) cycles or donor oocyte recipient cycles between morula and blastocyst embryos.
The goal of this retrospective cohort study was to determine if blastocyst embryo transfer yields better pregnancy outcomes compared to morula stage embryo transfer for fresh IVF, FET and in donor oocyte recipients.
Materials and Methods
We performed a retrospective analysis on a cohort of patients at a single fertility center with two providers in Staten Island, NY between January 1st, 2008 and December 31st, 2012. Medical records were reviewed for all1232 cycles. Donor oocyte recipient and FET cycles were analyzed separately. Cycles were excluded if no embryo transfer was performed (embryo banking, ovarian hyper stimulation syndrome, no embryos available for transfer), or if day 3 embryo transfer was performed secondary to poor prognosis. Only good prognosis patients were studied, defined as patient shaving at leastfour4-cell embryos on day 2. Each provider performed all oocyte retrievals and embryo transfers for their own patients without any cross coverage of procedures. One provider performed all embryo transfers for his good prognosis patients on day 4 at the morula stage, and the other provider performed all of his good prognosis transfers on day 5 at the blastocyst stage.
Ovarian stimulation was performed with hypothalamic down regulation with a GnRH agonist or with GnRH antagonist started with a lead follicle of 14 mm. Mixed protocols were used for all ovarian stimulation with FSH (either human derived or recombinant) and Menopur® (Ferring Pharmaceuticals, Switzerland). hCG10,000 IU was taken when at least 3 follicles over 18 mm were visible on transvaginal ultrasound. Oocyte retrieval was performed by transvaginal ultrasound-guided aspiration 35 hours after hCG administration. ICSI was performed in a standard fashion 4-6 hours after egg retrieval with fertilization check 16-18 hours later. Embryos were cultured in group culture in 25 μl micro drops Global® Total® media (Life Global®, USA) with a change of media on day 3.On days 2 and 3, embryos were scored according to the number and regularity of blastomeres, percentage of fragmentation, and multi nucleation. Embryo grading was assessed by a single embryologist. Blastocyst embryos were graded based on inner cell mass, trophectoderm, and fragmentation [2]. Morulas were graded based on Tao’s original criteria [8]. Embryo transfer was performed similarly by both physicians at the same location with no change in conditions during the study period.
Excess embryos were cryopreserved by vitrification (Life Global®, USA) on the day of embryo transfer. After thaw of frozen embryos for FET, embryos were held and warmed for approximately 4 hours prior to FET. FET cycles were performed using hypothalamic down regulation with leuprolide acetate followed by oral 17β-estradioluntil endometrial thickness reached at least 8 mm.
FSH Statistical analysis was performed with Stata v10 test for comparison between fresh morula and fresh blastocyst and morula transfers. P values of <0.05 were considered to be statistically significant. Baseline patient and cycle characteristics as well as pregnancy outcomes were analyzed by chi squared tests and t-test. Multivariable logistic regression was used to further characterize pregnancy outcomes. Two-way ANOVA was used to investigate the number of embryos transferred across cycle type and SARTage groups. Event rates over time were calculated and relative risk ratios were used to quantify clinical pregnancy and live birth relationships by cycle type exposure. There were no missing pregnancy outcome data.
This study was approved by the Staten Island University Hospital Institutional Review Board.
Results
A total of 1232 patients in the cohort underwent treatment. Of those, 101 had no embryo transfer (ovarian hyper stimulation syndrome, intentional embryo banking, or cancellation) and 227 had day 3 embryo transfers due to poor prognosis (< four 4-cell embryos on day 2). The remaining 904 patient cycles were included in the analysis –435IVF cycles, 378FET cycle, and 91donor egg recipient cycles.
Patient and cycle characteristics were similar between patients undergoing fresh blastocyst and morula embryo transfer (Table 1). Morula embryo transfers were performed exclusively by one provider and blastocyst embryo transfers by the other provider as described above. On average patients undergoing morula transfer had slightly more embryos transferred. This persisted throughout all SART age groups by two-way ANOVA (Table 2). Overall, implantation rates (38% v 22%, p<0.001), clinical pregnancy rates (51% v 38%, p<0.01), and live birth rates (42% v 31%, p=0.02) were higher for patients undergoing blastocyst embryo transfer compared to morula transfer. However on subgroup analysis, these benefits were seen exclusively in patients under age 35 (Figure 1). No other patients undergoing fresh, autologous embryos transfer had improvement in pregnancy outcomes with blastocyst transfer. Since there was a provider specific difference in the number of embryos transferred in all age groups, multivariable logistic regression models were created to adjust for number of embryos transferred, BMI, and endometrial thickness. In the adjusted model, clinical pregnancy rates (RR 1.65, p<0.01) and live birth rates (RR 1.65, p=0.01) again were only higher in patients under age 35 receiving blastocysts.
Characteristic
Fresh Blastocyst (n=205)
Fresh Morula (n=230)
p-value
Age (years)
35.1 ± 3.91
35.9 ± 4.92
0.07
FSH (mIU/mL)
6.94 ± 2.72
7.34 ± 3.06
0.15
BMI (kg/m2)
27.16 ± 6.42
28.31 ± 7.32
0.10
Endometrial Thickness (mm)
11.50 ± 3.12
11.31 ± 2.86
0.51
Peak Estradiol (pg/mL)
2369.56 ± 1317.33
2139.18 ± 1263.63
0.10
# Embryos Transferred
2.02 ± 0.69
2.92 ± 1.02
<0.01*
# Embryos Frozen
5.01 ± 5.01
4.46 ± 5.17
0.27
# Eggs Retrieved
13.78 ± 6.31
12.80 ± 6.17
0.10
Data represent mean ± standard deviation. T test was used for comparison. *denotes statistical significance.
Table 1: Patient and cycle characteristics for patients undergoing fresh, autologous blastocyst and morula embryo transfers.
Group
Fresh Morula
Fresh Blastocyst
P value
FET Morula
FET Blastocyst
P value
Age < 35
2.46 ± 0.82
1.75 ± 0.55
<0.01*
2.72 ± 0.92
2.23 ± 0.88
<0.01*
Age 35-37
2.74 ± 0.64
2.13 ± 0.67
<0.01*
2.65 ± 1.03
2.15 ± 0.97
0.03*
Age 38-40
2.98 ± 0.84
2.18 ± 0.58
<0.01*
3.35 ± 1.37
2.19 ± 0.7
<0.01*
Age 41-42
3.52 ± 0.85
2.75 ± 0.62
<0.01*
3.40 ± 0.84
2.52 ± 0.94
0.01*
Data represent mean ± standard deviation. Two-way ANOVA used for cross comparison.*denotes statistical significance. All age groups denoted similarly significant differences in the numbers of embryos transferred.
Table 2: Number of embryos transferred in autologous cycles by SART groups.
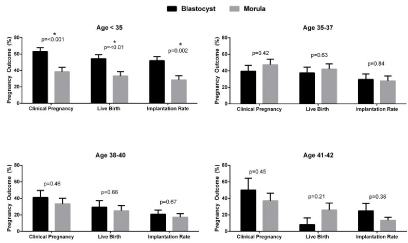
Figure 1: Pregnancy outcomes for fresh autologous cycles by SART groups. Data are analyzed by Chi squared test and are represented as mean ± standard
deviation. *denotes statistical significance.
FET cycles were analyzed in similar fashion. Patients had comparable endometrial thickness, estradiol levels, age, BMI and follicle stimulating hormone (FSH) levels (Table 3). Again there was a provider specific difference in the numbers of embryos transferred in all age groups by two-way ANOVA (Table 2). Among patients undergoing FET, there were no differences in implantation, clinical pregnancy, or live birth rates between blastocyst and morula embryo transfer in any of the SART age groups (Figure2). In subgroup analysis with multivariable logistic regression models adjusting for number of embryos transferred, BMI, and endometrial thickness, there were still no differences seen in clinical pregnancy or live birth rates in FET cycles (data not shown, all p>0.05).
Characteristic
FET Blastocyst (n=288)
FET Morula (n=90)
p-value
Age (years)
35.18 ± 4.31
35.96 ± 4.93
0.15
FSH (mIU/mL)
6.79 ± 4.13
7.27 ± 2.58
0.31
BMI (kg/m2)
28.01 ± 7.42
28.50 ± 7.83
0.59
Endometrial Thickness (mm)
10.62 ± 2.39
10.82 ± 2.54
0.51
Peak Estradiol (pg/mL)
430.93 ± 226.33
383.37 ± 199.39
0.08
# Embryos Transferred
2.22 ± 0.89
2.96 ± 1.08
<0.01*
Data represent mean ± standard deviation. T test was used for comparison. *denotes statistical significance
Table 3: Patient and cycle characteristics for patients undergoing autologous FET.
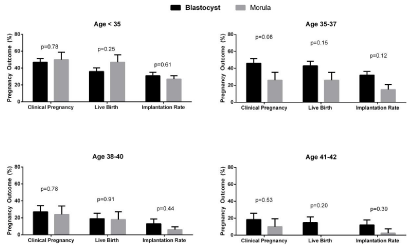
Figure 3: Pregnancy outcomes for autologous FET cycles by SART groups. Data are analyzed by Chi squared test and are represented as mean ± standard
deviation. *denotes statistical significance.
Among donor oocyte recipient cycles, there were no differences in age (40.8 v 41.9 years, p=0.57), BMI (27.8 v 30.5 kg/m², p=0.17), endometrial thickness (10.6 v 10.2 mm, p=0.56) peak estradiol in the recipient (404.9 v 450.7 pg/mL, p=0.47), or in the number of eggs retrieved (15.6 v 20.2, p=0.11). Donor egg recipients undergoing morula embryo transfer had a higher average number of embryos transferred (2.63 v 1.76, p<0.01). Comparable pregnancy outcomes were seen. Implantation rates (34% v 25%, p=0.50), clinical pregnancy rates (38% v 50%, p=0.37), and live birth rates (33% v 31%, p=0.87) were similar between blastocyst and morula embryo transfer. Using multivariable logistic regression and adjusting for BMI, age, and number of embryos transferred, day of transfer did not affect clinical pregnancy or live birth rate (p> 0.05).
There were no differences in the prevalence of multiple pregnancies born between blastocyst and morula embryo transfers in fresh cycles (26% v 28%, p=0.76), FET cycles (19% v 25%, p=0.52), or in donor oocyte recipient cycles (32% versus 25%, p=0.70). Rates of monozygotic pregnancies were also similar. There were four monozygotic pregnancies seen, all from fresh transfers, equally split between blastocyst and morula embryo transfers. No monozygotic pregnancies were seen in donor oocyte recipient cycles.
Conclusion
Blastocyst embryos yielded better pregnancy outcomes compared to morula embryo transfer only in patients under age 35 undergoing fresh autologous IVF cycles. Implantation rates, clinical pregnancy rates and live birth rates were all significantly higher in younger patients but not in any of the other SART groups. Morphological selection, while not perfect, is more likely to be advantageous in younger patients and may better predict euploidy. The prevalence of a euploidy even in young patients is surprisingly high [18]. In older patients the prevalence of a euploidy is so high that any morphological selection advantage at the blastocyst stage may be superseded by the sheer prevalence of a euploidy. These results are consistent with prior studies showing no clear advantage to blastocyst over cleavage stage embryo transfer in older patients [19,20].
Blastocysts did not improve any pregnancy outcomes after FET cycles. This is the first published data comparing blastocyst to morula FET. Cryopreservation and successful thaw may act as a different means of embryo selection, rather than awaiting blastocyst development. Morulas that successfully thaw for transfer may have inherently increased implantation potential, may represent embryos that would otherwise have developed into high quality blastocyst embryos, or may have a higher rate of euploidy.
Although three other studies have directly compared morula and blastocyst embryo transfer, this is the first study performed using modern extended media culture and vitrification. An additional strength of the study is that day of transfer was provider specific and not chosen for reasons of weekend planning or availability.
A weakness of our study is the higher average number of fresh and frozen embryos transferred at the morula stage. One provider, who performed all the morula embryo transfers, consistently transferred more embryos in patient of all ages, unrelated to prognosis and number of embryos available. This might have been expected to falsely elevate clinical pregnancy and live birth rates after morula transfer. As the data did not demonstrate this, advantages of blastocyst transfer could have been understated. Another weakness of our paper is its retrospective analysis. However, given the lack of experience with morula transfer at most centers and the increased prevalence of trophectoderm biopsy for pre-implantation genetic screening, we believe it is no longer feasible to perform a prospective trial in order to study morula transfer in good prognosis patients. Finally, although blastocysts did not improve pregnancy outcomes in our donor oocyte recipients, this subset of the study population was limited in number and more data are needed to confirm the findings.
References
- Buster JE, Bustillo M, Rodi IA, Cohen SW, Hamilton M, Simon JA, et al. Biologic and morphologic development of donated human ova recovered by nonsurgical uterine lavage. Am J Obstet Gynecol. 1985; 153: 211-217.
- Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998; 69: 84-88.
- Reynolds MA, Schieve LA, Jeng G, Peterson HB, Wilcox LS. Risk of multiple birth associated with in vitro fertilization using donor eggs. Am J Epidemiol. 2001; 154: 1043-1050.
- Wright V, Schieve LA, Vahratian A, Reynolds MA. Monozygotic twinning associated with day 5 embryo transfer in pregnancies conceived after IVF. Hum Reprod. 2004; 19: 1831-1836.
- Kanter JR, Boulet S, Kawwass JF, Jamieson DJ, Kissin D. Trends and correlates of monozygotic twinning after assisted reproductive technology. Obstetrics and gynecology. 2014; 123: 6S.
- El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril. 2013; 99: 632-641.
- Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009; 91: 305-315.
- Tao J, Tamis R, Fink K, Williams B, Nelson-White T, Craig R. The neglected morula/compact stage embryo transfer. Hum Reprod. 2002; 17: 1513-1518.
- Kyono K. The precise timing of embryo splitting for monozygotic dichorionic diamniotic twins: when does embryo splitting for monozygotic dichorionic diamniotic twins occur? Evidence for splitting at the morula/blastocyst stage from studies of in vitro fertilization. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2013;16: 827-832.
- Gianaroli L, Magli MC, Munné S, Fortini D, Ferraretti AP. Advantages of day 4 embryo transfer in patients undergoing preimplantation genetic diagnosis of aneuploidy. J Assist Reprod Genet. 1999; 16: 170-175.
- Grifo JA, Giatras K, Tang YX, Krey LC. Successful outcome with day 4 embryo transfer after preimplantation diagnosis for genetically transmitted diseases. Hum Reprod. 1998; 13: 1656-1659.
- Huisman GJ, Alberda AT, Leerentveld RA, Verhoeff A, Zeilmaker GH. A comparison of in vitro fertilization results after embryo transfer after 2, 3, and 4 days of embryo culture. Fertil Steril. 1994; 61: 970-971.
- Skorupski JC, Stein DE, Acholonu U, Field H, Keltz M. Successful pregnancy rates achieved with day 4 embryo transfers. Fertil Steril. 2007; 87: 788-791.
- Pantos K, Makrakis E, Chronopoulou M, Biba M, Perdikaris A, Dafereras A. Day 4 versus day 3 embryo transfer: a prospective study of clinical outcomes. Fertil Steril. 2008; 89: 573-577.
- Feil D, Henshaw RC, Lane M. Day 4 embryo selection is equal to Day 5 using a new embryo scoring system validated in single embryo transfers. Hum Reprod. 2008; 23: 1505-1510.
- Kang SM, Lee SW, Jeong HJ, Yoon SH, Koh MW, Lim JH, et al. Clinical outcomes of elective single morula embryo transfer versus elective single blastocyst embryo transfer in IVF-ET. J Assist Reprod Genet. 2012; 29: 423-428.
- Lee SH, Lee HS, Lim CK, Park YS, Yang KM, Park DW. Comparison of the clinical outcomes of day 4 and 5 embryo transfer cycles. Clin Exp Reprod Med. 2013; 40: 122-125.
- Munné S. Preimplantation genetic diagnosis for aneuploidy and translocations using array comparative genomic hybridization. Curr Genomics. 2012; 13: 463-470.
- Milki AA, Hinckley MD, Behr B. Comparison of blastocyst transfer to day 3 transfer with assisted hatching in the older patient. Fertil Steril. 2002; 78: 1244-1247.
- Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007; : CD002118.