
Research Article
Austin J Reprod Med Infertil. 2015;2(3): 1016.
Is there any Benefit in Adding LH Activity to the FSH Stimulation in Poor Responders?
Goswami M¹* and Nikolaou D²
1Assisted Conception Unit, Chelsea and Westminster Hospital, London, UK
2Assisted Conception Unit, Chelsea and Westminster Hospital, London, UK
*Corresponding author: Goswami M, Assisted Conception Unit, Chelsea and Westminster Hospital, London, UK
Received: April 27, 2015; Accepted: June 28, 2015; Published: June 30, 2015
Abstract
The aim of this study is to evaluate if there is any benefit in adding LH activity to the ovarian stimulation regime in poor responders. It is a retrospective study where all the IVF/ICSI cycles with poor response in 2012-2013 were analysed. The primary outcomes were clinical pregnancy rate per embryo transfer (CPR/ET) and live birth rate per embryo transfer (LBR/ET). The secondary outcomes were number of mature follicles, oocyte/follicle retrieval rate, endometrial thickness, failed fertilisation rate, implantation rate (IR). 9% of cycles in this period were deemed to have poor ovarian response. In 52 cycles, rFSH (group1), and in 25, hMG (group2) were used. There were no significant differences in mean age, AMH levels, sub fertility factors, and semen quality between the groups. There were no significant differences in the dose of FSH, duration of stimulation, protocols, number of mature follicles, endometrial thickness, oocyte retrieval rate/follicle and failed fertilisation rate between the groups. There were no significant differences in IR (19.5 versus 11.1, p=0.7), CPR/ET (14.6 versus 11.1, p=1), and LBR/ET (14.6 versus 0, p=016) between the 2 groups. We detected no significant differences in the primary or secondary outcomes with the use of rFSH or hMG in poor responders.
Keywords: Ovarian stimulation; Poor ovarian response; LH activity
Abbreviations
E2: Estradiol; FSH: Follicle Stimulating Hormone; ESHRE: European Society of Human Reproduction and Embryology; POR: Poor Ovarian Response; IVF: In Vitro Fertilisation; hMG: Human Menopausal Gonadotropin; LH: Luteinising Hormone; rFSH: Recombinant Follicle Stimulating Hormone; rLH: Recombinant Luteinising Hormone; ICSI: Intra Cytoplasmic Sperm Injection; AMH: Anti Mullerian Hormone; GnRHa: Gonadotropin Releasing Hormone Agonist; s.c.: Sub Cutaneous; rhcg: Recombinant Human Chorionic Gonadotropin; CPR: Clinical Pregnancy Rate; LBR: Live Birth Rate; SPSS: Software Package for Statistical Analysis; IBM: International Business Management Corporation; MERIT: Menotrophin versus Recombinant FSH in vitro Fertilisation Trial
Units: Standard International system of units has been used in this article.
Introduction
Dealing with poor ovarian response poses a clinical challenge in assisted reproduction. Clinicians have been striving to overcome this problem by innovating various treatment strategies, with an aim to reduce the risks of cycle cancellation and poor pregnancy rates in this group [1-4].
Regarding the incidence of poor ovarian response, there is a wide range (5-24%) quoted in the literature [5-6].
The definition of poor ovarian response also varies in the literature. As reported by Pandian et al [7], there has been a wide range of definition of poor response used in various studies, including less than 2 to 5 follicles, less than 3 to 6 oocytes retrieved, maximum E2 levels attained during stimulation (100-660 pg/ml), total dose and duration of gonadotropin used; in a background of known markers of poor ovarian response, such as 40 years or older, and high serum FSH [8]. The ESHRE consensus group has defined poor ovarian response, when at least two of the following three features are present: (i) advanced maternal age or any other risk factor for POR; (ii) a previous POR; and (iii) an abnormal ovarian reserve test; or two previous episodes of POR despite maximal stimulation [9].
Regarding the optimum stimulation regime in IVF in general (i.e. not specifically in poor responders), there is longstanding debate and controversy regarding whether use of hMG, or supplementing LH to rFSH, confers any benefit in ovarian stimulation cycles [10- 12]. The Cochrane review in 2003 [13], a subsequent meta-analysis [14], and the BFS Practice Committee recommendation [15] suggest that there is no significant difference in clinical outcome in terms of oocyte numbers, pregnancy and live birth rates, with hMG and rFSH use. Similarly, no significant difference in ongoing pregnancy or live birth rates with use of hMG or rFSH was noted in a more recent metaanalysis [16]. Coomarasamy et al [17] showed in their meta-analysis however that hMG use in comparison to rFSH, was associated with significant increase in live birth rates in down regulation protocol. On the other hand, the meta-analysis by Lehert et al [18] gave evidence of fewer oocytes retrieved and higher dose of gonadotropin required, when hMG is used, in comparison to rFSH. However, there were comparable pregnancy rates in both the groups. Similarly, Anderson et al found lower number of oocytes retrieved, but higher level of E2 with use of hMG in comparison to rFSH, but no significant difference in ongoing pregnancy rates in the 2 groups [19].
Specifically for ovarian stimulation for poor responders, there is some suggestion in the literature that using hMG instead of recombinant FSH, or LH supplementation in the stimulation protocol, is associated with better ovarian response and higher live birth rates in group of women poorly responding to FSH alone [20- 22, 15]. However, this claim is also fraught with conflicting evidence as other studies or reviewers performing meta-analysis found no significant differences following LH supplementation to rFSH, use of hMG in comparison to rFSH [2, 23, 5, 24]. Bosch et al found a significant increase in implantation rates and on-going pregnancy rates with rLH supplementation to FSH in the 36-39 years age group of women, in comparison to rFSH use alone, but there was no such benefit noted in the younger age group [25].
Therefore, it seems that the evidence in the literature of clinical outcome following the use of hMG versus rFSH, or following LH supplementation to rFSH during controlled ovarian stimulation is conflicting , not only in an unselected population, but also in the poor responder category of women.
A Cochrane review in 2010 concluded that there is insufficient evidence to support the use any particular intervention of ovarian stimulation or adjuvant therapy in poor response, and more robust data were needed [7]. A more contemporary meta-analysis regarding the supplementation of rLH in poor responders was also inconclusive for dearth of consistent definition of poor response used in the studies, and the limited number of studies included in the meta-analysis [5].
In the light of this evidence, we embarked on this study on our local population, aiming to evaluate if there is any difference in clinical outcome with the use of hMG (Menopur), which has both FSH and LH activity, versus rFSH (Gonal F) in our poor responders. It is acknowledged that hMG exerts LH activity and being a urinary derivative, is contaminated with other impurities as well; and hence this study does not reflect a direct translation of efficacy of LH per se.
Materials and Methods
It is a retrospective study where all the IVF/ICSI cycles in the two years period from January 2012 to December 2013 in a tertiary care hospital were analysed. In this study, the definition of poor ovarian response was taken as the yield of less than 4 oocytes, following the use of 3000 or more units of FSH, as described by Kailasam et al [26]. The inclusion criteria included all the cycles in this time period that reached oocyte retrieval, where 0 to 3 oocytes were retrieved following use of a total of 3000 or more units of FSH. Egg donor cycles and embryo freezing cycles with no fresh transfer were excluded. The type of ovarian stimulation protocol and the type of gonadotropin used depended on the discretion of the individual clinical practitioners.
The poor responder women were identified from the electronic database, and the relevant information regarding the patients and the IVF/ICSI cycles, were obtained from the database. The confounding factors potentially impacting the clinical outcome including age, cause of infertility, AMH level were noted.
The types of intervention during the IVF/ICSI cycles were noted; including the type of ovarian stimulation protocol used (down regulation/ flare / antagonist), type of gonadotropin used, total dose and duration of stimulation, type of ovulation trigger used, semen quality, IVF/ ICSI procedure, any difficulty during embryo transfer, number of embryos transferred, type of luteal phase support.
The cycles were analysed in 2 groups, depending on the use of Gonal F (group 1) or Menopur (group 2), and the confounding factors were compared, in order to ascribe any difference in clinical outcome, related to the use of rFSH or hMG.
The primary outcomes analysed were CPR and LBR, and the secondary outcomes analysed were number of mature pre-ovulatory follicles (=/> 17mm), highest E2 level, number of oocytes retrieved, fertilisation and failed fertilisation rates, endometrial thickness achieved, implantation rates, miscarriage rates and frozen embryo rates.
Analysis was performed using SPSS 21(IBM software). Normality of data was tested using Shapiro Wilk, and Kolmogorov Smirnov tests for the 2 groups as appropriate, and parametric or non-parametric test were done as necessary. Comparison of continuous variables was made by using Independent sample T test. Pearson’s chi squared test was used in case of nominal variables.
Results
In the down regulation protocol, GnRHa, Buserelin acetate 0.5 mg was used s.c. from day 21 to achieve down regulation, and the same was started in the early follicular phase for the flare protocol. In the antagonist cycles, Cetrorelix 0.25 mg (Cetrotide, Merck Serono) was used subcutaneously from day 5/6 to prevent endogenous LH surge. Ovarian stimulation was achieved using either hMG (Menopur, Ferring), or rFSH (Gonal F, Merck Serono), administered by daily s.c. injections and the doses were altered depending on the serial USS and serum E2 levels. Ovulation was triggered when the leading follicles reached 18mm, using rhcg injection (Ovitrelle, Merck Serono), and 250 mcg. Luteal phase support was provided using Progesterone vaginal pessaries, 400 mg twice a day (Cyclogest, Actavis).
In the 2 year period, there were 851 IVF/ICSI cycles in this unit, 77 (9%) of which were deemed to have POR, by the criteria used. In 52 cycles, rFSH (group1) was used, and in 25 cycles, hMG (group2) was used for ovarian stimulation.
There were no significant differences in the variables including the mean age, AMH levels, sub fertility factors (p=0.16), and semen quality on the treatment day (p=0.21) in the 2 groups, as shown in Table 1.None of these women had a history of previous live birth. (22)42.3% cycles in group 1 were antagonist using Cetrorelix, (21)40.3% were long down regulation and (9)17.3% were agonist flare protocols. This was not statistically different (p=0.16) to that in group 2, where (21)84% were antagonist cycles, and the rest (4), 16% long down regulation. Ovulation trigger and luteal phase support were identical in the 2 groups. The proportion of cycles treated by ICSI was comparable in the 2 groups, with slightly more ICSI treatments in group 2 (13 ICSI out of 25 in group 2, versus 22 out of 52 in group 1) although it was not statistically significant.
rFSH (group 1)
hMG (group 2)
p
Mean age
39.3(S.D.4.43)
38.7(S.D. 3.82)
0.6
Median AMH
2.9 (S.D 4.66)
2 (S.D 1.36)
0.29
Median dose of FSH units
4,500 (S.D 1277.9)
4500(S.D1498.7)
0.9
Median duration of stimulation days
12 (S.D 2.96)
12 (S.D 3.69)
0.9
ICSI %
42.3
52
0.47
Median no. of mature follicles (=/>17mm)
2 (S.D 1.53)
3 (S.D 1.45)
0.38
Endometrial thickness mm
10 (S.D 2.47)
10.5 (S.D 2.04)
0.36
Median no. of mature oocytes
2 (S.D 0.74)
2 (S.D 0.71)
0.74
Mature oocyte retrieval rate/follicle %
100
66.6
0.7
Fertilisation rate %
100
66.6
0.7
No. of embryos transferred
1(S.D 0.85)
1 (S.D 0.89)
0.9
IR%
19.5 (8/41)
11.1 (2/18)
0.7
CPR/ET%
14.6 (6/41)
11.1 (2/18)
1
LBR/ET%
14.6 (6/41)
0 (0/18)
0.16
Failed/abnormal fertilisation rate%
21.2 (11/52)
28 (7/25)
0.57
Embryo freezing rate%
2.44 (1/41)
5.55 (1/18)
0.5
Table 1: Demonstrates the characteristics, interventions and outcome comparisons in the 2 groups.
Table 1 confirms that there were no significant differences in the median dose of gonadotropin used, and the duration of stimulation days in the 2 groups. There were no significant differences in the number of mature follicles, endometrial thickness, and mature oocyte retrieval/follicle rate, number of mature oocytes retrieved, fertilisation rates and failed or abnormal fertilisation rates in the 2 groups.
41 cycles in group 1 and 18 in group 2 progressed to embryo transfer, with 9.8% (4/41) difficult transfers in group 1, and 5.6% (1/18) difficult transfers in group 2, which was not statistically significant (p=1).
There were 8 and 2 implantations, 6 and 2 clinical pregnancies, and 6 and 0 live births in the 2 groups respectively. There was 11.1% (2/18) miscarriage rate per embryo transfer in group 2, and none in group 1, and no difference in embryo freezing rate in the 2 groups.
Thus, in this study there was no significant difference in either the primary, or the secondary outcomes with the use of rFSH or hMG in poor responders.
Figure 1 depicts the comparison of clinical pregnancy rates in the 2 groups.
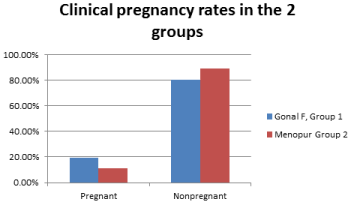
Figure 1: Demonstrates the comparison of the clinical pregnancy rates in the
2 groups using rFSH (group 1), versus hMG (group 2).
Discussion
The main strengths of this study are that: i) a validated evidence based definition of poor ovarian response has been used based on the criteria of Kailasam et al [26], where poor ovarian response was taken as the yield of less than 4 oocytes, following the use of 3000 or more units of FSH. In this retrospective analysis, this patient population also fulfil the ESHRE criteria of poor responders in having a previous poor response, and low AMH, as a marker of reduced ovarian reserve [9]. This is important, since one of the main weaknesses of recent meta-analysis was inconsistency in the definitions used for poor response in the various studies [5]; ii) In the previous studies and meta-analyses comparing the clinical outcome following the use of rFSH versus hMG, either solely GnRH agonist down regulation protocol [24], GnRH flare protocol [11], GnRH agonist down regulation and flare protocols [5], or only GnRH antagonist protocol [23] have been used. This study reports the outcome following GnRH agonist down regulation protocol, flare protocol, as well as GnRH antagonist protocol, and shows that there was no significant difference in clinical outcome with either rFSH or hMG. This finding is significant as it had been postulated that the drastic inhibition of LH secretion in antagonist cycles would necessitate exogenous LH supplementation [23, 27]. There is also report of lower number of oocytes and reduced fertility rates following the use of rFSH devoid of LH activity, in GnRHa down regulation cycles [23,28]. However, this claim is also fraught with controversy, as there are reports that in down regulated cycles, the level of endogenous LH activity is sufficient to maintain follicular development and pregnancy [29,23,5]. The argument for using gonadotropin with LH activity stems from the two cell-two gonadotropin hypothesis, which emphasises that both FSH and LH are synergistically important in folliculogenesis. FSH acts by promoting aromatase activity to convert androgen to 17 ß E2 in granulosa cells, and LH drives the theca cells to produce the substrate for the aromatase activity, androgens [2, 30-32]. The validity of this hypothesis is clearly demonstrated when inducing ovulation in the gonadotropin deficient World Health Organisation Type 1 anovulatory population. It was proposed that supplementation of LH in poor responders would be beneficial effect on oocyte quality and fertilisation [5, 33]. Ferraretti et al [21] had hypothesised that in poor responders, rLH supplementation was required to restore the diminished oocyte competence, as opposed to simply increasing the dosage of FSH alone, to improve IR and CPRs. In the Cochrane review, Mochtaret al [2004] also propounded that poor responders with a higher risk of miscarriages may benefit from rLH supplementation, related to reduction of early pregnancy miscarriages. On the contrary, inappropriate high levels of LH during folliculogenesis could potentially lead to follicular atresia and impaired oocyte development [5, 35, 36].
However, in this study we selected women who responded poorly but had egg collections, which was our end point. Starting from this, we went back to see which women had been on FSH Alone and which had been on FSH and LH-activity. We found that women who responded poorly were just as likely to have used FSH alone or FSH and LH activity together.
There was no significant difference in the primary outcome, LBR, with the use of rFSH or hMG in this study. In comparison to the reported studies, this is similar to the findings of other studies or meta-analyses which reported comparable LBR/ ongoing pregnancy rates with the use of rFSH versus hMG, or with LH supplementation to rFSH [5,14, 18]. The MERIT group established non-inferiority of hMG in producing on-going pregnancy rates, although it’s superiority over rFSH was not established [19]. Our findings were different to that of the results of the meta-analysis by Coomarasami etal [17], who found a 4% increase in LBR with the use of hMG in agonist down regulation cycles.
This study did not find any significant difference in CPR and IR between the 2 groups; and this was corroborated by other studies [2, 23].
There was no significant difference in the oocyte numbers between the 2 groups; which was similar to the findings in other studies reporting their experience in poor responders [2, 23, 5]. In contrast, Lehert et al and the MERIT trial reported more oocyte numbers in the rFSH group [18, 19]. Interestingly, the increased oocyte numbers in the rFSH group in the MERIT trial was not associated with improved oocyte quality in that group. On the contrary, they found more number of top quality embryos in the hMG group. It has been proposed that improved oocyte quality is mediated by the LH activity of hMG, mediated by action on cumulus cells [19, 37]. In this study however, there was no difference in the maturity of the oocytes in the 2 groups.
Barranetxea et al [2] reported no difference in serum E2 levels in the 2 groups, in a population of poor responders; which is similar to the findings of this study. On the contrary, Anderson et al [19] had reported achievement of higher serum level of E2 in the rFSH group. They however did not find any difference in the endometrial thickness, and in the proportion of women with triple layer structure of the endometrium; which was corroborating with the findings of this study, with no difference in the endometrial thickness in the 2 groups. The MERIT group [19] although found an association of hyper echogenic endometrium in women treated with rFSH, which they hypothesised, could be relevant in treatment outcome.
Lehert et al [18] found higher total dose of Gonadotropin required in the hMG group, unlike the findings of this study. However, Chung et al [23] reported no difference in the cumulative dosage of FSH requirement, or the duration of stimulation needed to attain follicle maturity in between the 2 groups of poor responders <40 years old. A few other papers have also reported no difference in the duration of stimulation in between the 2 groups, which was similar to the findings of this study [2, 5].
Conclusion
Thus in this study, the two groups of women with poor response, who used FSH-stimulation only versus FSH and LH-activity combined, were similar with comparable AMH levels, identical demographic features including similar age groups, sub-fertility factors and no previous live births. Both the groups had IVF/ ICSI treatments with GnRHa down regulation protocol, flare protocol or antagonist protocol, using rFSH or hMG. All the confounding factors and interventions being comparable, there were no significant differences in the IR,CPR, LBR, as well as in the mature follicle and oocyte numbers, embryo freezing rates, endometrial thickness and maximum serum E2 achieved, or in the dose and duration of FSH stimulation required. Accepting the main drawback of the inherent limitation associated with a retrospective study, the main strengths of this study lie in the use of validated definition of POR, and the simultaneous reporting of the outcome of the commonly used treatment protocols.
Acknowledgements
The authors thank the nursing and embryology staff of the Assisted Conception Unit at the Chelsea and Westminster Hospital, who helped in identifying the cases and collection of data. Last but not the least, we thank the patients of this unit whose anonymised data have been used in this study.
References
- Akman MA, Erden HF, Tosun SB, Bayazit N, Akoy E, Bahceci M. Comparison of agonistic flare-up-protocol and antagonistic multiple dose protocol in ovarian stimulation of poor responders: results of a prospective randomized trial. Hum Reprod .2001; 16: 868–870.
- Barrenetxea G, Agirregoikoa JA, Jimeenez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. FertilSteril. 2008; 89: 546 –553.
- Dragisic KG, Davis OK, Fasouliotis SJ, Rosenwaks Z. Use of a luteal estradiol patch and gonadotropin-releasing hormone antagonist suppression protocol before gonadotropin stimulation for in vitro fertilization in poor responders. FertilSteril. 2005; 84: 1023–1026.
- Olivennes F, Cunha-Filho JS, Fanchin R, Bouchard P, Frydman R. The use of GnRH antagonists in ovarian stimulation. Hum Reprod Update. 2002; 8: 279-290.
- Fan W, Li S, Chen Q, Huang Z, Ma Q, Wang Y. Recombinant Luteinizing Hormone supplementation in poor responders undergoing IVF: a systematic review and meta-analysis. Gynecol Endocrinol. 2013; 29: 278-284.
- Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol. 1997; 104: 521-527.
- Pandian Z, McTavish AR, AucottL, Hamilton MPR, Bhattacharya. Interventions for ’poor responders’ to controlled ovarian hyper stimulation (COH) in in-vitro fertilisation (IVF) (Review). The Cochrane Library. 2010; 1: CD004379.
- Surrey ES, Schoolcraft WB. Evaluating strategies for improving ovarian response of the poor responder undergoing assisted reproductive techniques. Fertil Steril. 2000; 73: 667-676.
- Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011; 26: 1616-1624.
- Agrawal R, Holmes J, Jacobs HS. Follicle-stimulating hormone or human menopausal gonadotropin for ovarian stimulation in in vitro fertilization cycles: a meta-analysis. Fertil Steril. 2000; 73: 338-343.
- Barrenetxea G, Agirregoikoa JA, Jimeenez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. FertilSteril. 2008; 89: 546–553.
- Caglar GS, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hasani S. Recombinant LH in ovarian stimulation. Reprod Biomed Online. 2005; 10: 774-785.
- Van Wely M, WestergaardLG, Bossuyt PM, Van der Veen F. Human menopausal gonadotropin versus recombinant follicle stimulation hormone for ovarian stimulation in assisted reproductive cycles. Cochrane Database Syst Rev. 2003; 1; CD003973.
- Kolibianakis E M, Kalogeropoulou L, Griesinger,G, Papanikolaou ,EG, Papadimas J, Bontis, J, et al. Among patients treated with FSH and GnRH analogues for in vitro fertilization, is the addition of recombinant LH associated with the probability of live birth? A systematic review and meta-analysis. Hum Reprod Update. 2007; 13: 445-452.
- Nardo L G, Bosch E, Lambalk C B, Gelbaya T A. Controlled ovarian hyperstimulation regimens: a review of the available evidence for clinical practice. Produced on behalf of the BFS Policy and Practice Committee. Hum Fertil. 2013; 16: 144-150.
- Jee BC, Suh CS, Kim YB, Kim SH, Moon SY. Clinical efficacy of highly purified hMG versus recombinant FSH in IVF/ICSI cycles: a meta-analysis. Gynecol Obstet Invest. 2010; 70: 132-137.
- Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van Wely M. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysis. Hum Reprod. 2008; 23: 310-315.
- Lehert P, Schertz JC, Ezcurra D. Recombinant human follicle-stimulating hormone produces more oocytes with a lower total dose per cycle in assisted reproductive technologies compared with highly purified human menopausal gonadotropin: a meta-analysis. ReprodBiolEndocrinol. 2010; 16: 112.
- Andersen AN1, Devroey P, Arce JC . Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006; 21: 3217-3227.
- De Placido G , Alviggi C , Perino A , Strina I , Lisi F , Fasolino A , et al. Italian Collaborative Group on Recombinant Human Luteinizing Hormone . Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod. 2005; 20: 390-396.
- Ferraretti AP, Gianaroli L, Magli MC, D'angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. 2004; 82: 1521-1526.
- Phelps JY, Figueira-Armada L, Levine AS, Vlahos NP, Roshanfekr D, Zacur HA, et al. Exogenous luteinizing hormone (LH) increases estradiol response patterns in poor responders with low serum LH concentrations. J Assist Reprod Genet. 1999; 16: 363-368.
- Chung K, Krey L, Katz J, Noyes N. Evaluating the role of exogenous luteinizing hormone in poor responders undergoing in vitro fertilization with gonadotropin-releasing hormone antagonists. FertilSteril. 2005; 84: 313-318.
- Musters AM, van Wely M, Mastenbroek S, Kaaijk EM, Repping S, van der Veen F, et al. The effect of recombinant LH on embryo quality: a randomized controlled trial in women with poor ovarian reserve. Hum Reprod. 2012; 27: 244-250.
- Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. FertilSteril. 2011; 95: 1031-1036.
- Kailasam C, Keay SD, Wilson P, Ford WC, Jenkins JM. Defining poor ovarian response during IVF cycles, in women aged <40 years, and its relationship with treatment outcome. Hum Reprod. 2004; 19: 1544-1547.
- Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001; 179: 39-46.
- Fleming R, Lloyd F, Herbert M, Fenwick J, Griffiths T, Murdoch A. Effects of profound suppression of luteinizing hormone during ovarian stimulation on follicular activity, oocyte and embryo function in cycles stimulated with purified follicle stimulating hormone. Hum Reprod. 1998; 13: 1788-1792.
- Balasch J, Vidal E., Penarrubia J, Casamitjana R, Carmona F, Creus M et al. Suppression of LH during ovarian stimulation: analysing threshold values and effects on ovarian response and the outcome of assisted reproduction in down-regulated women stimulated with recombinant FSH. Hum Reprod. 2001; 16: 1636-1643.
- Couzinet B, Lestrat N, Brailly S, Forest M, Schaison G. Stimulation of ovarian follicular maturation with pure follicle-stimulating hormone in women with gonadotropin deficiency. J Clin Endocrinol Metab. 1988; 66: 552-556.
- [No authors listed]. Recombinant human luteinizing hormone (LH) to support recombinant human follicle-stimulating hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study. The European Recombinant Human LH Study Group. J Clin Endocrinol Metab. 1998; 83: 1507-1514.
- Shoham Z, Balen A, Patel A, Jacobs HS. Results of ovulation induction using human menopausal gonadotropin or purified follicle-stimulating hormone in hypogonadotropic hypogonadism patients. Fertil Steril. 1991; 56: 1048-1053.
- Loutradis D, Vomvolaki E, Drakakis P. Poor responder protocols for in-vitro fertilization: options and results. Curr Opin Obstet Gynecol. 2008; 20: 374-378.
- Mochtar MH, Van der Veen, Ziech M, van Wely M. Recombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database Syst Rev. 2007; : CD005070.
- Peñarrubia J, Fábregues F, Creus M, Manau D, Casamitjana R, Guimerá M, et al. LH serum levels during ovarian stimulation as predictors of ovarian response and assisted reproduction outcome in down-regulated women stimulated with recombinant FSH. Hum Reprod. 2003; 18: 2689-2697.
- Tarlatzis B, Tavmergen E, Szamatowicz M, Barash A, Amit A, Levitas E, et al. The use of recombinant human LH (lutropin alfa) in the late stimulation phase of assisted reproduction cycles: a double-blind, randomized, prospective study. Hum Reprod. 2006; 21: 90-94.
- Platteau P, Smitz J, Albano C, Sørensen P, Arce JC, Devroey P. Exogenous luteinizing hormone activity may influence the treatment outcome in in vitro fertilization but not in intracytoplasmic sperm injection cycles. Fertil Steril. 2004; 81: 1401-1404.