
Research Article
Austin J Reprod Med Infertil. 2015;2(3): 1017.
Oxidative DNA Damage in Male Germ Cells in Normozoospermic Infertile Men: A Case for Concern
Mishra SS¹, Kumar S¹, Singh G¹, Mohanty K¹, Vaid S¹, Malhotra N³, Singh N³, Kumar R² and Dada R¹*
1Laboratory for Molecular Reproduction and Genetics, Department of Anatomy, All India Institute of Medical Sciences, New Delhi, India
2Deptartment of Urology, All India Institute of Medical Sciences, New Delhi, India
3Deptartment of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India
*Corresponding author: Dada R, Laboratory for Molecular Reproduction and Genetics, Department of Anatomy, All India Institute of Medical Sciences (AIIMS), New Delhi-110029, India
Received: May 04, 2015; Accepted: June 15, 2015; Published: June 28, 2015
Abstract
Background: Sperm DNA is highly susceptible to oxidative damage due to various endogenous/exogenous factors and deficient DNA damage detection and repair mechanisms. Telomeres are the favourable targets for oxidative damage. So, this study was planned to evaluate seminal oxidative stress, sperm DNA damage and sperm telomere length in normozoospermic infertile men.
Material and Method: The study included 30 infertile men and 30 controls. Reactive oxygen species (ROS) estimation was done by chemiluminesence method and 8-isoprostane (8-IP) and 8-hydroxy-2-deoxy-guanosine (8-OHdG) levels by ELISA. The average sperm telomere length was measured using quantitative Real Time PCR method. Sperm Chromatin Structure Assay (SCSA) was done by flow cytometry.
Results: Mean ROS (89.43±36.32 Vs. 15.04 ± 10.81 RLU/sec/million sperm; p=0.016) and 8-IP levels (698.7 ± 127.8 Vs. 278.02 ± 72.03 pg/ml; p=0.035) were significantly elevated in cases as compared to controls. 8−OHdG levels were higher in patients (25.33 ± 13.34 pg/ml) as compared to controls (15.34 ± 8.3 pg/ml) (p= 0.032). Mean DNA Fragmentation Index (DFI %) in patients was 40.31 ± 14.83, which was higher as compared to controls (24.43 ± 8.83) (p<0.0001). Mean telomere length was significantly (p=0.012) lower in patient group (0.737 ± 0.038) as compared to fertile control group (0.787 ± 0.054).
Conclusion: Normozoospermic infertile men may experience seminal oxidative stress, DNA damage and telomere shortening. Oxidative stress, loss of sperm DNA integrity, accumulation of mutagenic bases, telomere shortening may lead to genome hyper mutability and may be the underlying aetiology of male infertility. As majority of causes leading to oxidative stress are modifiable/ treatable (sedentary life style, excessive alcohol intake, smoking, excessive use of cell phone, acute or chronic infections, varicocele), management of oxidative stress may minimize free radical levels and subsequent oxidative damage to sperm DNA. Thus sperm with its limited antioxidant/DNA repair capacity may benefit by adopting a policy of prevention is better than cure.
Keywords: Oxidative stress; Sperm DNA damage; Telomere; Infertility
Introduction
Infertility is a health problem affecting approximately 15% of all couples trying to conceive. It is now evident that in at least 50% of all cases, reduced semen quality is a factor contributing to the problem. In 20% of the couples, the main cause is solely male related, and in another 27%, both partners contribute to the problem. Male infertility can be the result of congenital or acquired urogenital abnormalities, infections of the genital tract, varicocele, endocrine disturbances, genetic or immunological factors. However, in at least 50% of the infertile men, no explanation to their reduced semen quality can be found. Recent studies have shown that alterations in the sperm molecular factors (paternal genome, mitochondrial DNA and transcripts) may be the underlying cause of infertility [1-3]. It is considered to be a complex lifestyle disease involving interplay of over 2000 genes and several lifestyle and environmental factors.
In past decade there has been a secular trend of decline in semen quality, concomitant with increase in male genitourinary abnormalities and testicular cancer. In recent years apart from an increase in number of cases with idiopathic azoospermia or oligozoospermia there has been a significant increase in numbers of cases with normal sperm parameters experiencing infertility and recurrent spontaneous abortions. Such cases with unexplained infertility need to be evaluated beyond the routine semen analysis as per WHO guidelines 1999. As about 1-3% of children each year are conceived via ART, the need for in depth analysis of sperm factors, such as free radical levels and assessment of integrity of sperm DNA is important. Use of sperm with oxidative DNA damage may impact health trajectory of child.
Studies have shown that sperm DNA integrity assessment can be applied in ART [4] in order to find the most effective treatment in a couple and counsel couple regarding management [5-7]. Semen quality is known to be influenced by a variety of lifestyle, environmental, and occupational factors. Although still much is unknown, the origin of sperm DNA damage is believed to be multifactorial where defects during spermatogenesis, abortive apoptosis and oxidative stress may be possible causes of a defective sperm DNA, but the damage is believed to be mainly oxidative [8].
Human sperm DNA is highly susceptible to oxidative damage. The most common types of DNA damage include single or double DNA strand breaks chemical modifications of DNA bases and inter and intra strand crosslinks. There are several factors that lead to sperm DNA damage. They include alterations in chromatin modelling during the process of spermiogenesis, abortive apoptosis, supra physiological levels of reactive oxygen species (ROS), and activation of caspases and endonucleases [7, 9]. Oxidative stress damages both mitochondrial and nuclear DNA. Mitochondrial DNA accumulates mutation and nuclear DNA shows fragmentation, denaturation and accumulation of highly mutagenic oxidized adducts like 8-hydroxy-2-deoxy guanosine (8-OHdG) [3]. Sperm cells are exposed to oxidative stress during transport and storage through the male reproductive tract and oxidative stress is believed to be major cause of DNA damage and sperm function [10]. Physiological levels of oxidative stress are essential for normal sperm function but levels of ROS exceeding the physiological limits cause sperm DNA damage. Advanced paternal age, storage in the epididymis, acute and chronic infections, chemotherapy, varicocele, sedentary life style and obesity are the risk factors associated with increased oxidative damage in sperm DNA. Unhealthy life style habits like smoking, alcohol consumption, sedentary and stressful lifestyle, occupational exposure to hazardous chemicals like lead, phthalates, organophosphorous compounds and environmental exposures to insecticides, pesticides, electromagnetic radiation, and high temperature exposure are other factors that increase ROS levels and consequently induce damage to the sperm DNA [11]. Presence of immature germ cells, morphologically abnormal spermatozoa and inflammatory cells in semen, varicocele are endogenous causes of excess free radical production [12]. The role of oxidative stress is now a well established aetiology of infertility and poor semen quality [11, 13, 14]. The important nucleic acid oxidation product includes 8-OHdG which is used as a oxidative DNA damage marker. 8-OHdG is a base adduct formed due to oxidation of guanine nucleotide found maximally in telomeric DNA in sperm nuclear periphery in the nucleohistone compartment. Telomeres are preferential targets of oxidative stress. Sperm with longer telomeres help to maintain critical telomere length during cleavage, which aids in maintaining species-specific telomere length in the newborn [15]. Studies on telomerase null mice have also shown that when these mice fertilized wild-type oocytes resulted in decreased fertilization and blastocyst formation, increased embryo fragmentation, and apoptosis [16]. Therefore, it may be possible that sperm with shortened telomere length may not respond to oocyte signals for pronucleus formation even if fertilization does occur. This may result in impaired cleavage, which may result in poor quality blastocyst, increased apoptosis, or failed implantation. Telomeric DNA is guanine rich and more susceptible to oxidative stress. If there is attrition in telomere or there is a double strand break in the ends of telomere, it recruits DNA repair enzymes or it activates DNA damage response pathways. Telomeres block unwanted DNA repair reactions and avoid detection by the DNA damage signaling pathway. So, telomeric damage or shortening may result in recognition of the chromosome ends as double strand breaks and recruitment of DNA damage response mechanisms [17]. Estimating the levels of 8-OHdG in the seminal plasma of the infertile patients is an efficient and reliable method to analyse oxidative damage to the sperm nucleus [18].
Recent studies from our lab have documented sperm DNA damage in infertile men with normal semen parameters and also in male partners of couples experiencing pre and post implantation losses [2]. Persistence of DNA damage may be due to limited DNA repair mechanisms Sperm a highly polarised, transcriptionally inert cell has only base excision repair (BER) but lacks APE and XRCC1. There may be altered or reduced expression of DNA damage detection and repair enzymes in infertile patients [14]. Kumar et al, 2015 documented that simple life style modification, adopting meditation and yoga practice for a minimum of 6 months can significantly reduce sperm DFI and levels of 8-OHdG. The group further documented that fathers of cases with non familial sporadic heritable retinoblastoma have elevated ROS levels and oxidative sperm DNA damage [19]. Majority of these men had advanced age (>35 yrs) and were smokers. As DNA damage is chiefly oxidative in nature and arises due to various life style factors, majority of which are modifiable, the integrity of sperm genome can be maintained by following a healthy lifestyle and this may have great impact on offspring health and can significantly reduce incidence of childhood morbidity and even children cancer.
Therefore in this study we have measured oxidative stress levels to understand aetiology of DNA damage, which was quantified by levels of ROS and 8 Isoprostane (8-IP) and oxidative DNA damage by estimating the levels of 8-OHdG. We have also measured telomere length in these normozoospermic infertile patients and controls to know the effect of oxidative stress on the telomere length. Since oxidative stress induced sperm DNA damage is chiefly produced by numerous endogenous and exogenous factors majority of which are modifiable, adoption of a healthy life style and prompt management of infections, inflammations and varicocele can minimize / prevent oxidative damage to sperm DNA.
Materials and Methods
The study was initiated after institutional ethical clearance and written informed consent from patient and controls. The female partners of all these cases were normal after complete clinical, gynecological, hormonal and radiological examination. Human ejaculates were obtained from 30 healthy volunteers of proven fertility from family planning OPD of Obstetrics and Gynecology department, and 30 male partners of couples experiencing primary infertility within age group of 18-45 years. A detailed family history was recorded in a pre-designed proforma. Cytogenetic analysis has been done for all cases to exclude cases with abnormal chromosome complement. All cases with recent history of fever, drug intake, any inflammatory disorders, and infections were excluded. Semen analysis was assessed by World Health Organization (1999) criteria. These patients after through clinical examination were referred from the Department of Gynecology and Obstetrics and Department of Urology, AIIMS, New Delhi. Statistical analysis was done using Student t test and Pearson correlation.
Semen analysis
Semen analysis was done twice at 2 weeks interval. Samples were collected after minimum of 48 hours and not longer than 7 days of sexual abstinence. The name of the patient, period of abstinence and time of collection were recorded on the form accompanying each semen analysis. Samples were collected in the privacy of room near the laboratory and were delivered to the laboratory within one hour after collection. The samples were obtained by masturbation and ejaculated into a clean, wide-mouthed glass or plastic container. The procedure for sample collection was explained to the patients and controls. Semen analysis was done as per WHO guidelines (1999).
ROS detection by chemiluminescence assay in neat semen
The ROS production in 400 μl of liquefied neat semen was measured after addition of 10 μl of 5 mM solution of luminol in DMSO (dimethylsulphoxide, Sigma Chemical Co.). A tube containing 10 μl of 5 mM luminol (5-amino-2,3-dihydro- 1,4-phthalazinedione, Sigma Chemical Co., St. Louis, MO, USA) solution in DMSO used as a blank. Chemiluminescence was measured in duplicate for 10 min using the Berthold detection luminometer (USA). Sample analysis was done along with blank, positive control (H2O2+PBS+Luminol) and negative control (PBS+luminol). Results were expressed in relative light units (RLU) per second and per 1 × 106 spermatozoa.
Sperm chromatin structure assay
Preparation of samples: The SCSA was performed according to the procedure described by Evenson et al, 2005 [20]. The aliquot from each ejaculate was thawed in a water bath at 37ºC for 30 seconds and diluted to a concentration of 2×106 sperm/mL in TNE buffer to a total of 200 mL in a falcon tube. Immediately, 0.4 mL of acid detergent solution (0.08 mol/L HCl, 0.15 mol/L NaCl, 0.1% v/v Triton X-100, pH 1.2) was added to the Falcon tube. After exactly 30 seconds, 1.2 mL of AO-staining solution (6 mg AO [chromatographicall purified: Polysciences, Inc. USA] per mL citrate buffer [0.037 mol/L citric acid, 0.126 mol/L Na2HPO4, 1.1 mmol/L EDTA disodium, 0.15 mol/L NaCl, pH 6.0]) was added. For every 6 test samples, 1 standard reference sample was analyzed to ensure instrument stability.
Flow cytometric measurements: The samples were analyzed using a FAC Scan flow cytometer (BD Biosciences), with an air cooled argon laser operated at 488 nm and a power of 15 mW. The green fluorescence (FL1) was collected through a 515-545 nm band pass filter, and the red fluorescence (FL3) was collected through a 650 nm long pass filter. The sheath/ sample was set on ‘‘low,’’ adjusted to a flow rate of 200 events/s when analyzing a sample containing 2 ×106 sperm/mL. Immediately after the addition of the AO staining solution, the sample was placed in the flow cytometer and run through the flow system. All the samples were assessed in duplicate at 1-month interval and the average was taken. After complete analysis of the sample, the X-mean (red fluorescence) and Y-mean (green fluorescence) values were recorded manually after selecting gate for sperm cells using FlowJo software (Oregon). Strict quality control was maintained throughout the experiment.
DFI Calculation: Post-acquisition DFI calculation was performed offline in the Flowjo software. The sperm cells are gated after excluding debris and high DNA stain ability (HDS) cells and mean values of red and green fluorescence were recorded manually. The DFI was then calculated by the formula, DFI = mean red fluorescence/ (mean red fluorescence + mean green fluorescence).
8 Isoprostane (8-IP) estimation: 8 Isoprostane levels were estimated by ELISA. The quantification was done by Cayman’s 8-IP EIA Kit. Protocol was followed as described by the manufacturer for the quantification.
8-Hydroxy-2-deoxy-Guanosine (8-OHdG) estimation: For this assay sperm DNA was isolated. Then it was digested by DNase1 and Alkaline phosphatase. The quantification of 8-OH-dG was achieved by using Cayman’s 8-OHdG EIA kit. Protocol was followed essentially as described by the manufacturer for the quantification of 8-OH-dG (Promega).
Telomere length estimation
Real time PCR: Sperm telomere length was determined from the sperm DNA by a quantitative real-time PCR-based method as described elsewhere [15]. Briefly, the relative mean telomere length was determined by comparing the value from absolute quantification of telomere DNA with a single copy reference gene, 36B4 (T/S ratio). These two assays were carried out as separate reactions on separate plates maintaining the sample positions between the two plates. Amplification signals were quantified by the standard curve method using a DNA template series (100 ng, 10 ng, 1 ng, 0.1 ng, 0.01 ng/microlitre) on every plate. All randomized DNA samples (20 ng) and standard dilution was processed as triplicates on 96-well plates using Bio-Rad CFX 96 (Her- cules, CA, USA). The purpose of the standard curve was to assess and compensate for inter-plate variations in PCR efficiency. Amplification of the telomeric repeat region was expressed relative to amplification of 36B4, a single copy gene (SCG) encoding acidic ribosomal phosphor protein located on chromosome 12. Real time kinetic quantitative PCR determines, for each sample well, the Ct, i.e., the fractional cycle number at which the well’s accumulating fluorescence crosses a set threshold that is several standard deviations above baseline fluorescence. A plot of Ct versus log (amount of input target DNA) is linear, allowing simple relative quantisation of unknowns in comparison to a standard curve derived from amplification, in the same plate, of serial dilutions of a known reference DNA sample. For this study, telomere (T) PCRs and SCG PCRs were always performed in separate 96-wellplates.
Statistical analysis: Pearson (χ2/Fisher’s exact test) was applied to make a comparison between two groups. P-values less than 0.05 were considered as significant. All tests were done using STATA 11.2 software for windows.
Results
The sperm parameters like sperm count (p=0.026) and forward motility (p=0.0002) were significantly lower in infertile men compared to controls and no significant difference in the seminal volume and pH was observed between infertile men and controls. Out of 30 cases, 22 men (73.33%) had normal semen parameters as per WHO 1999 guidelines.
ROS levels
The seminal ROS level (RLU/sec/million sperm) were significantly higher (p=0.016) in infertile men (89.43±36.32 RLU/sec/million sperm) as compared to fertile controls (15.04 ± 10.81 RLU/sec/ million sperm). Out of 30 cases 17 cases (56.67%) had ROS > 22 RLU/ sec/million sperm and 13 cases (43.33%) had < 22 RLU/sec/million sperm, out of 30 controls 21 controls (70%) had ROS <22RLU/sec/ million sperm and 9 (30%) had > 22RLU/sec/million sperm (Figure 1).
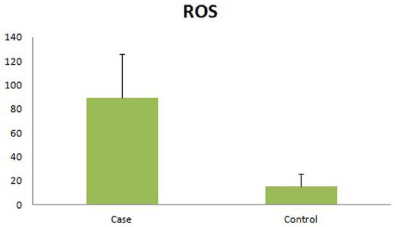
Figure 1: Seminal ROS levels in patients and controls, expressed as RLU/
sec/million sperm 8-OHdG Level.
The total 8-OHdG level was measured in DNA isolated from the spermatozoa after removal of somatic cells. The 8-OHdG levels were significantly higher in the patients (25.33 ± 13.34 pg/ml) as compared to controls (15.34 ± 8.3 pg/ml) (p= 0.032) (Figure 2).
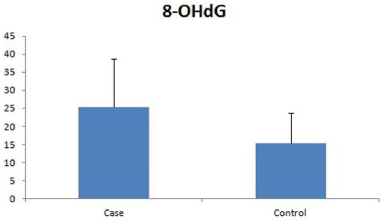
Figure 2: Seminal 8-OHdG levels in patients and controls, expressed as pg/
ml.
DNA fragmentation index
The mean DFI (%) in infertile men was found to be 40.31 ± 14.83, which was higher as compared to controls (24.43 ± 8.83). This difference in DFI between cases and controls was statistically significant (p<0.0001). Out of 30 cases 19 cases (63.33%) had >30 % DFI and 11 cases (36.67%) had < 30% DFI, in controls 23 (75.9%) men had <30% DFI and 12 (24.1%) had>30% DFI (Figure 3).
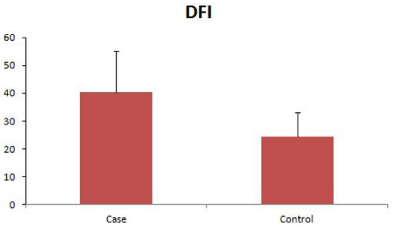
Figure 3: Sperm DFI levels in patients and controls, expressed as %DFI.
8-Isoprostane level
The seminal 8-IP levels were found to be significantly higher in the cases (698.7 ± 127.8 pg/ml) as compared to the controls (278.02 ± 72.03 pg/ml) (p=0.035) (Figure 4).
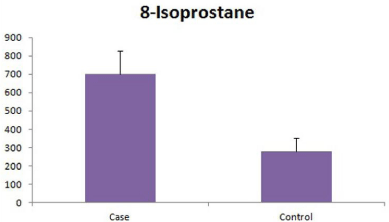
Figure 4: Seminal 8-IP levels in patients and controls, expressed as pg/ml.
Telomere length
Relative quantification of telomere length was done by real time PCR. The average telomere length in infertile men was 0.737 ± 0.038, significantly (p=0.012) less than fertile control 0.787 ± 0.054 (Figure 5).
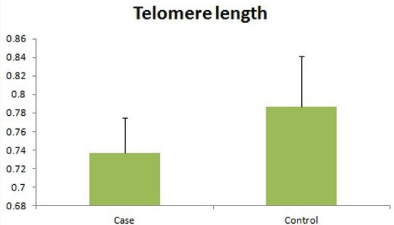
Figure 5: Relative telomere length in the patients and controls.
Discussion
Though 30 to 40% people in the reproductive age group have a qualitative defect in sperm production but there are cases with normal sperm parameters with unexplained infertility. In this study 22 infertile men (73.33%) had normal semen parameters. Such men with normal semen parameters may have high levels of DNA damage (DNA fragmentation and denaturation) [21] and about 25% normozoospermic infertile men have elevated free radical levels and experience oxidative stress. Several endogenous and exogenous factors induce oxidative stress and majority of these factors are modifiable and thus elevated free radical levels can be minimised by simple life style modifications e.g. cessation of smoking, excess alcohol intake, minimize exposure to insecticide, pesticide, decrease duration of cell phone usage, prompt treatment of infections, inflammations ad engaging in moderate physical activity and thereby minimize oxidative DNA damage. This suggests that sperm DNA damage is a factor which can be associated with the aetiology in a substantial proportion of idiopathic and unexplained infertility patients [22]. This damage is chiefly oxidative as indicated by raised levels of 8-OHdG, 8-IP and is in concordance with previous studies [23, 24]. Presence of oxidative DNA damage in sperm with limited or lower levels of DNA damage repair factors may also be the aetiology of elevated incidence of gonadal/extragonadal neoplasms in infertile men. As these men also have low levels of PARP1 thus the mutagenic load in germ cells is higher [14]. Oxidative stress, sperm DNA damage and short telomere are hallmarks of ageing, so we hypothesize that infertility may be due to accelerated testicular ageing. Elevated 8-OHdG levels and accumulation of this mutagenic base adduct in patients may be the cause of pre and post implantation losses, congenital malformations and cancers in childhood [2]. It may also lead to accumulation of mutations in sperm DNA Oxidative stress is associated with upregulation in levels of DNA methyl transferases (DNMTs) and aberrant methylation in genes associated with schizophrenia and bipolar disorder may be the cause of increased incidence of these disorders and autism in children borne by fathers with aberrant sperm molecular factors. In this study we found higher levels of 8-OHdG and ROS in infertile patients as compared to fertile controls. Elevated ROS levels were further validated by elevated 8-IP levels, a lipid peroxidation product, produced during oxidation of sperm membranes which is a stable marker of lipid peroxidation as compared to MDA (malonaldehyde) the levels of which vary depending on nutritional intake of lipids.
Recent studies in our lab has shown elevated oxidized DNA adducts and DNA damage in fathers with advanced age (>35 years) and men who smoked. Kumar and associate [19, 25] have also documented that practice of meditation and yoga are therapeutic for oxidative DNA damage, and seminal ROS levels declined following 10 days of yoga practice and sperm DFI declined significantly after 6 months of yoga practice. Thus adopting a healthy life style can have profound impact in lowering oxidative DNA damage and thus improve sperm DNA integrity.
As sperm has highly truncated repair mechanism due to low levels of PARP1, sperm DNA damage persists [14]. Since sperm has minimal cytoplasm and thus a limited anti oxidant capacity, raised free radical levels produced by dysfunctional mitochondria directly damage PUFA rich sperm membrane and nuclear DNA [13]. Damage to sperm membrane affects sperm motility. This acts as a biological safeguard and prevents transmission of damaged DNA to ova. However, the use of such sperm with damaged DNA in ART is a matter of great concern as it may increase the burden of cases with childhood morbidity, neuropsychotic disorders and cancer and affect lifelong health of the offspring. We have also measured telomere length and found shorter telomere length in these men possibly because telomeres are preferential targets of oxidative stress. Presence of shorter telomere in sperm results in DNA repair reactions which results in single and double stranded breaks [26] and also chromosomal aberrations which may result in infertility. In a previous study from our lab we have documented lower TAC levels in semen of infertile men. Also infertile couples both the male and female partner experience psychological stress and are usually depressed. An ongoing study from our lab on depression has shown that can with depression have higher cortisol levels and raised levels of inflammatory markers IL6, TNFapha and raised free radical levels which could further induce oxidative injury to sperm genome. However practice of meditation and yoga caused significant decline in levels of cortisol, IL6 and upregulation of endorphins, telomerase activity and anti aging gene sirtuins. Upregulation of telomerase acivity is highly significant because it indicates the maintainence of telomere length. Oxidative and psychological stress cause accelerated attrition of telomeres and thus accelerate aging of cell which may be slowed by upregulation of this key enzyme for telomere length maintainence [25].
Sperm DNA damage may be the major cause of ART failure, even in spontaneous conception with sperm cells with damaged DNA can lead to implantation losses, poor embryonic development, congenital malformations and even spontaneous abortions [2, 24, 27]. Sperm DNA integrity assay is a good diagnostic test in investigation and treatment of infertility. Sperm DNA integrity assessment in combination with the WHO semen parameters are required to achieve better results in ART programmes and prevent pre and post implantation losses. Assessment of sperm DNA integrity becomes more important in ART as in this process of assisted reproduction the sperm experiences oxidative stress during sperm washings making the sperm genome more vulnerable to oxidative DNA damage [28, 29]. Life style habits like smoking, alcohol consumption, extremes of physical activities, junk food and environmental exposures to electromagnetic radiations from cell phones, exposure to pesticides and organophosphates and phthalates are exogenous causes of excess free radical levels and may be the aetiology of DNA damage and infertility in cases of yet unexplained infertility in normozoospermic men. Since poor life style habits is a major predisposing factor for oxidative DNA damage in sperm, simple life style modifications like adopting meditation and yoga in daily practice has proven to be highly efficient in reducing oxidative stress (10 days) and DNA damage (3-6 months of practice) [19].
Oxidative stress not only damages the DNA but also the sperm RNA. Though sperm is transcriptionally and translationally inert it has several transcripts and non coding RNA which are regulators of promoters of genes which are of critical developmental importance. Kumar K et al, 2012 have documented altered sperm transcripts levels in sperm of normozoospermic men [2]. This may also be the cause of pre and post implantation losses, and congenital malformations. As sperm DNA damage is not completely repaired due to inefficient repair mechanisms (lacks APE and XRCC1) it depends on oocyte for repair of damaged DNA. In infertile couples especially those opting for ART the age of couples is usually advanced (28-39 years) and in such cases the genetic factors/repair mechanisms in oocyte may also be suboptimal and may not be able to repair entire DNA damage resulting in persistence of DNA damage. As the fertilised oocyte enters the S phase this damage manifests in every cell of the zygote and may be the cause of recurrent spontaneous abortion, congenital malformations and childhood cancers. Recent studies from our lab on non familial sporadic heritable retinoblastoma [19] have documented significantly elevated ROS levels, DNA damage and 8-OHdG in sperm of fathers who had children with non familial retinoblastoma. Majority of these men were smokers and had normal sperm count, emphasizing the role of unhealthy life style and social habits on germ cell quality. These men also had advanced paternal age (>35 years) and sperm DFI showed a positive correlation with paternal age. Thus this study highlights the role of decline in sperm quality due to oxidative damage to sperm genome and thus tests for analysis of sperm DNA integrity should be included in evaluation of male infertility especially in men with normal sperm parameters.
Acknowledge
The authors acknowledge financial support from DBT and ICMR.
References
- Kumar M, Kumar K, Jain S, Hassan T, Dada R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics (Sao Paulo). 2013; 68 Suppl 1: 5-14.
- Kumar K, Deka D, Singh A, Chattopadhyay P, Dada R. Expression pattern of PRM2, HSP90 and WNT5A in male partners of couples experiencing idiopathic recurrent miscarriages. J Genet. 2012; 91: 363-366.
- Shamsi MB, Govindaraj P, Chawla L, Malhotra N, Singh N, Mittal S, et al. Mitochondrial DNA variations in ova and blastocyst: implications in assisted reproduction. Mitochondrion. 2013; 13: 96-105.
- Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, et al. A survey of small RNAs in human sperm. Hum Reprod. 2011; 26: 3401-3412.
- Aitken RJ, Sawyer D. The human spermatozoon--not waving but drowning. Adv Exp Med Biol. 2003; 518: 85-98.
- Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014; 29: 2402-2412.
- Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011; 18: 1005-1013.
- Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010; 25: 2415-2426.
- Zini A, Libman J. Sperm DNA damage: clinical significance in the era of assisted reproduction. CMAJ. 2006; 175: 495-500.
- Moazamian R, Polhemus A, Connaughton H, Fraser B, Whiting S, Gharagozloo P, et al. Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod. 2015; 21: 502-515.
- Aitken RJ, Finnie JM, Muscio L, Whiting S, Connaughton HS, Kuczera L, et al. Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum Reprod. 2014; 29: 2136-2147.
- Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010; 132: 728-730.
- Shamsi MB, Venkatesh S, Kumar R, Gupta NP, Malhotra N, Singh N, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010; 47: 38-43.
- Mishra S S, Kumar R, Malhotra N, Dada R. Expression of PARP1 in primary infertility patients and correlation with DNA fragmentation index a pilot study. JASI. 2013; 62: 90-104.
- Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013; 287: 803-807.
- Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell. 2001; 12: 2023-2030.
- de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol. 2010; 75: 167-177.
- Guz J, Gackowski D, Foksinski M, Rozalski R, Zarakowska E, Siomek A, et al. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS One. 2013; 8: e68490.
- Kumar S, Gautam S, Tolahunase M, Chawla B, Yadav R K, Kumar P, et al. Improvement in sperm DNA quality following simple life style intervention: A study in fathers of children with non familial sporadic heritable retinoblastoma. J Clin Case Report. 2015; 5: 509.
- Evenson DP, Wixon R. Comparison of the Halosperm test kit with the sperm chromatin structure assay (SCSA) infertility test in relation to patient diagnosis and prognosis. Fertil Steril. 2005; 84: 846-849.
- Venkatesh S, Dada R. Acridine orange binding to RNA interferes DNA fragmentation index calculation in sperm chromatin structure assay. Fertil Steril. 2010; 94: e37, author reply e38.
- Mayorga-Torres BJ, Cardona-Maya W, Cadavid Á, Camargo M. [Evaluation of sperm functional parameters in normozoospermic infertile individuals]. Actas Urol Esp. 2013; 37: 221-227.
- Aitken J, Krausz C, Buckingham D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994; 39: 268-279.
- Mishra S S, Kranthi V, Kumar R, Malhotra N, Mohanty K, et al. Oxidative Damage to Sperm DNA: Clinical Implications. Andrology Open Access. 2014; 3: 1.
- Kumar S, Yadav R, Yadav R K, Tolahunase M, Dada R. Telomerase activity and cellular aging might be positively modified by a yoga-based lifestyle intervention: A case report. J Altern Complement Med. 2015; 21: 370-372.
- Moskovtsev SI, Willis J, White J, Mullen JB. Disruption of telomere-telomere interactions associated with DNA damage in human spermatozoa. Syst Biol Reprod Med. 2010; 56: 407-412.
- Vemparala K, Kumar M, Mishra SS, Dada R. Role of sperm transcripts in the etiology of idiopathic recurrent pregnancy loss. Andrology. 2014 April; 2: Supp 1.
- Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. 2011; 13: 69-75.
- Thilagavathi J, Venkatesh S, Kumar R, Dada R. Segregation of sperm subpopulations in normozoospermic infertile men. Syst Biol Reprod Med. 2012; 58: 313-318.